6 : Density of nitrogen at N.T.P. is 1.25 gm/litre. Calculate R.M.S. speed of nitrogen molecules at 127°C. (Atmospheric pressure = %3D 1.013 x 10 N/m2)
Q: Vcom 13
A: (a) The rotational inertia of the solid sphere. (b)the frictional force(f) between the incline and ...
Q: D4.1. Given the electric field E =(8xyza, +4x za, - 4x ya-) V/m, find the differen- tial amount of w...
A:
Q: Consider the figure of an equilateral triangle of side d moving with a velocity v in a direction per...
A: Here L is transformed length and L0 is our original length. The length which is along the velocity w...
Q: What is the electric field at the midpoint M of the hypotenuse of the triangle shown below? (Assume ...
A: Solution; First of all, find the distance of the 2q charge from point M. Use the angle bisector theo...
Q: For the below Casing Tally, fill the missing numbers given that the Shoe will be set at 2550 ft. CUM...
A: The coloumns of this table representing the following values are calculated or measured as: JT NO: ...
Q: A body of mass m falls from the rest in a medium offering resistance proportional to the square of t...
A: A body which shall fall from rest has initial velocity equals to 0m/s. Using this and the equations ...
Q: An airplane moves 140 m/s as it travels around a vertical circular loop which has a 1.0-km radius. W...
A: Given data, Speed of the airplane = 140 m/s Radius of the circle = 1 km Mass of the pilot = 70 kg
Q: Consider two identical systems A and B are connected to each other. At constant volumes V and V3, th...
A:
Q: 2. A block of mass m rests on a plane inclined at 0 with the horizontal. The block is attached to a ...
A: Solution:-Given that Free Body diagram of block:- (FBD-1)
Q: 2. What angle in radians is subtended by an arc of length 78.54 cm on the circumference of a circle ...
A: Circumference is the perimeter of the circle. It is the arc length of the circle if it is opened to ...
Q: You can find the temperature inside your refrigerator without putting a thermometer inside. Take a c...
A: Given that, T(0.5)=45°F T(1)=55°FT Room temperature =70o We know that, dv/dT=K(T0−T) Equatio...
Q: Object A is released from rest at height h. Two seconds later object B is released from rest at the ...
A: Given, Initial velocity of both object is zero. initial height of both object is h And object B fall...
Q: fyou have two instruments used to measure a temperature of 45 C inside the factory. However, the two...
A:
Q: (a) Discuss the propagation of field vectors E and H in a conducting medium and show that their spee...
A:
Q: (Q) Consider a system of three noninteracting particles that are confined to move in a one-dimension...
A: energy of the particle inside energy level n is given by E=n2π2h22mL2 where m is the mass of the par...
Q: What is the maximum charge on the capacitor of an L-C circuit, where, L = 85.0 mH and C = 3.20 uF. D...
A: Given that, Inductance, L=85×10-3 H Capacitance, C=3.2×10-6 F Maximum current, I0=8.5×10-3 A Angular...
Q: Bolum-7+A2B1C3uUxiD5ViBM A5.00-kg block is moving at vro.00 m/s along a frictionless, horizontal sur...
A: When the spring is compressed, the kinetic energy of spring is converted into potential energy of sp...
Q: te 93 (a) W = U = 41 42 -e 4TE0\13 23. 4περ 2α
A: Given: Work done and energy as, W=U=q34π∈0q1r13+q2r23=+e4π∈0-e2a++ea To find: Solution of the above ...
Q: Only Handwritten Solution Required. showing its vanny. 21. Give two physical examples of Einstein's ...
A: Note : We don't provide Handwritten Solutions. Sorry for inconvenience. The mass-energy equivale...
Q: A tuning fork B produces 8 beats per second with another tuning fork C of frequency 512 Hz. When the...
A: In this question we have to find the original frequency of fork 'B' we know the initial number of be...
Q: A cube with edge lengths 1.6 m contains positively charged particles. Consider a Gaussian surface of...
A: Given, Edge length a =1.6 mElectric field E=750 N/C
Q: A ballet dancer spins about a vertical axis at 90 r.p.m. with arms outstretched. With the arms folde...
A: Given that, Initial frequency (n1) = 90 r.p.m. ...
Q: 17. What is the importance of Michelson-Morley experiment? Discuss its negative results.
A: Michelson-Morley Experiment: It was a scientific experiment to test for the presence and properties ...
Q: A body of mass m falls from the rest in a medium offering resistance proportional to the square of t...
A: Given,mass =mResistance Fr=kv2k=proportionality constantFrom equation of motion,mdvdt=mg-Frmdvdt=mg-...
Q: Two wheels of moment of inertia 4 kg m? rotate side by side at the rate of 120 rev | min and 240 rev...
A: As the wheels are initially moving in opposite direction, their angular speed are in opposite direct...
Q: ldentify the equation given below and discuss in detail the theorem pertaining to the equation given...
A: The given equation is F = Q4πεo ∑ k =1N(Qk(r-rk)|(r-rk)|3)where Q is the test chargeQk is the syste...
Q: 10 - Bölüm 9- soru-9 A car of mass m moving at a speed y = 20.0 m/s collides and couples with the ba...
A: Answer : If a mass m1 with velocity v1collides inelastically with a mass m2 with velocity v2 , then...
Q: What is the law of conservation of linear momentum? Establish this law with the help of Galilean inv...
A:
Q: Explain with suitable examples the meaning of inertial and non-inertial frame of reference. Explain ...
A:
Q: A system has N weakly interacting identical particles. Each particle has 2 energy levels that are 0 ...
A: To answer: The energy U of this system at a given temperature T The value of energy as T→∞ and T→0
Q: A wire having young's modulus 1.2 x 1011 N/m2 is subjected to the stress of 2.4 x 107 N/m2. If the l...
A: Given data : Young's modulus (Y)=1.2×1011 N/m2 Tensile stress Mgπr2=2.4×107 N/m2...
Q: A coin kept on a horizontal rotating disc has its center at a distance of 0.1 m from the axis of rot...
A: In this question we have to find the angular speed ω . In this case the distance of the centre of th...
Q: 1. The force on a charged particle (with charge q) moving with a velocity u(F) in the presence of an...
A: Concept used: The no. of test particles required depends on no. of independent components required t...
Q: When a cold alcohol thermometer is placed in a hot liquid, the column of alcohol goes down slightly ...
A: Thermometer are device used for measurement of temperature and within it different liquid are us...
Q: Find the current in the 10,5 and 2 ohm resistor 10 Q 2Ω. 5 Ω (4)12 A 3 A ww ww
A:
Q: 50 : Find the decrease in the volume of 4 liters of water when subjected to a pressure of 8 atmosphe...
A: Given : V=4 liters=4×10-3 m3K=2×109 N/m2dP=8 atms=8×1.013×105 N/m2
Q: For applying the method of joint at joints the forces need to be concurrent Select one: True False
A: True
Q: A certain wheel has a rotational inertia of 12 kg-m?. As it turns through 5.0 rev its angular veloci...
A: The following data are given: Rotational Inertia, I=12 kgm2 No of revolution=5 rev Initial Angular V...
Q: A parallel plate capacitor has charge q and plate area A. A dielectric materia of dielectric constan...
A:
Q: A wire 6.50 m long with diameter of 2.05 mm has a resistance of 0.0290 Ω. What material is the wire ...
A: Length of wire L = 6.50 m Diameter of wire D = 2.05 mm = 2.05 × 10- 3 m Resistance of wire R = 0.029...
Q: 56 : The lower surface of metal cube, having each side of length 10 cm, is kept fixed and tangential...
A: In the given question we have to find the magnitude of force applied (F). We have the modulus of rig...
Q: A circular disc of mass M and radius R is rotating about its axis with angular speed ω1 . If another...
A:
Q: A rigid bar, pinned at O, is supported by two identical springs as shown in Fig1. Each spring consis...
A: Given: d=3/4in, R=2.5in, τmax=20 ksi, n=18 Refer to the free body diagram below : Shearing stress ...
Q: 3. To conserve energy, room temperatures are kept at 68.0°F in the winter and 78.0°F in the summer. ...
A: To convert Fahrenheit to Celsius, we use T(°C) = (T(°F) - 32) * (5/9) For winter, room te...
Q: Reduce the circuit to a single resistor at terminals a-b Show the complete solution with new drawing...
A: The resistance of the resistor are given as, R1=5 Ω, R2=8 Ω, R3=20 Ω, and R4=30 Ω. TO DETERMINE: (a...
Q: said to be space-like and when it is time-like ? What is meant by space-time separation between two ...
A: We will first look at the concept of distance between two points in ordinary 3 dimensional space, ...
Q: 16 : Write down the equation of simple harmonic progressive wave of amplitude 0.05 m and period 0.04...
A: A simple harmonic progressive wave is a wave which continuously advances in a given direction withou...
Q: 5. The surface temperature of the Sun is about 5750 K. Wha is this temperature on the Fahrenheit sca...
A: Given: Temperature of Sun in kelvin is 5750 K. To find: Temperature on Fahrenheit scale.
Q: A copper wire of length 25 m has a resistance of 0.55 Q at 20°C. a) What is the radius of the wire? ...
A: Resistance of an object is defined as the opposition to the current flow in the material. It depends...
Q: Please help with the solution. Give an example of when you’d use a combined plane change vs a si...
A: Concept used: The change in inclination of orbit of orbiting body is called orbital inclination chan...
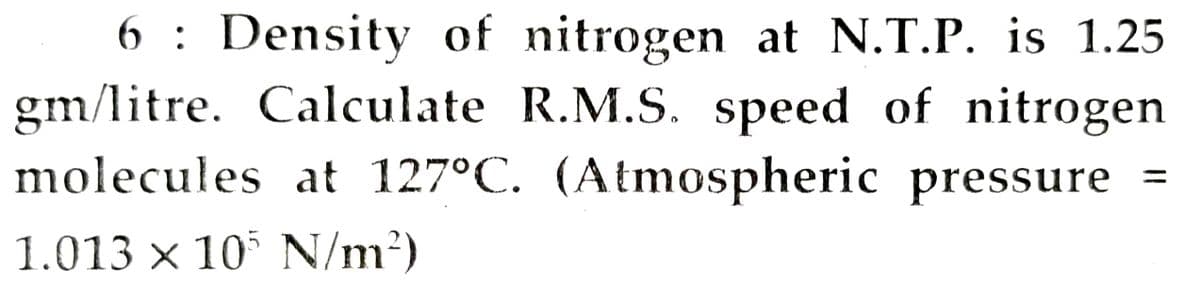
Step by step
Solved in 2 steps
