6. A basketball is inflated to a pressure of 1.90 atm in a 23.0°C garage. What is the pressure of the basketball outside where the temperature is 0.00°C?
6. A basketball is inflated to a pressure of 1.90 atm in a 23.0°C garage. What is the pressure of the basketball outside where the temperature is 0.00°C?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
please show work for question #6
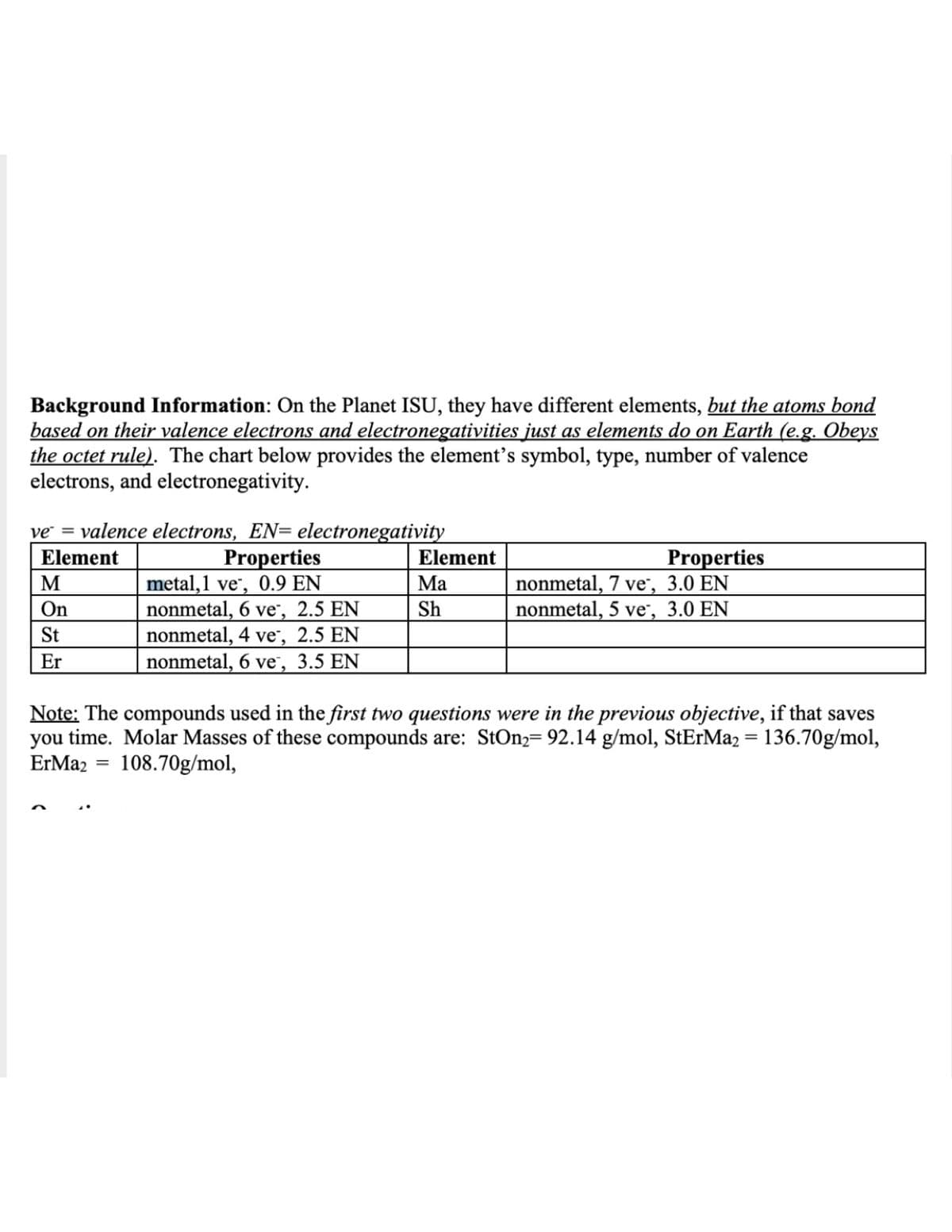
Transcribed Image Text:Background Information: On the Planet ISU, they have different elements, but the atoms bond
based on their valence electrons and electronegativities just as elements do on Earth (e.g. Obeys
the octet rule). The chart below provides the element's symbol, type, number of valence
electrons, and electronegativity.
ve = valence electrons, EN= electronegativity
Properties
metal,1 ve", 0.9 EN
nonmetal, 6 ve, 2.5 EN
nonmetal, 4 ve, 2.5 EN
nonmetal, 6 ve, 3.5 EN
Element
Element
Properties
nonmetal, 7 ve", 3.0 EN
nonmetal, 5 ve, 3.0 EN
M
Ma
On
Sh
St
Er
Note: The compounds used in the first two questions were in the previous objective, if that saves
you time. Molar Masses of these compounds are: StOn2= 92.14 g/mol, StErMaz = 136.70g/mol,
ErMa2
108.70g/mol,
%3D

Transcribed Image Text:3. Explain why a balloon filled with He on a cold winter's day, shrinks when you take it to
your car to take it home from the store. Your explanation should include a description of
the behavior of the gas particles. You may draw pictures to help your explanation if you
want, but it is not required.
4. A balloon filled with helium gas at 20°C occupies 4.91 L at 1.00 atm. The balloon is
immersed in liquid nitrogen at -196°C, while the pressure is raised to 5.20 atm. What is
the volume of the balloon in the liquid nitrogen?
5. Determine the volume, in L, of H,S (at 102°C and 1.20 atm) needed to produce 45.5 g of
S. Assume that there is excess SO, present.
2 H,S(g) + SO,(g) ·
3 S(s) + 2 H,O(g)
6. A basketball is inflated to a pressure of 1.90 atm in a 23.0°C_garage. What is the pressure
of the basketball outside where the temperature is 0.00°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The