7–102_A liquid delivery system is being designed such that ethylene glycol flows out of a hole in the bottom of a large tank, as in Fig. P7–100. The designers need to predict how long it will take for the ethylene glycol to completely drain. Since it would be very expensive to run tests with a full-scale prototype using ethylene glycol, they decide to build a one-
7–102_A liquid delivery system is being designed such that ethylene glycol flows out of a hole in the bottom of a large tank, as in Fig. P7–100. The designers need to predict how long it will take for the ethylene glycol to completely drain. Since it would be very expensive to run tests with a full-scale prototype using ethylene glycol, they decide to build a one-
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter5: Analysis Of Convection Heat Transfer
Section: Chapter Questions
Problem 5.70P
Related questions
Question
100%
Can anyone show me how did they manage to get the 45.8 degree Celsius by interpolation?
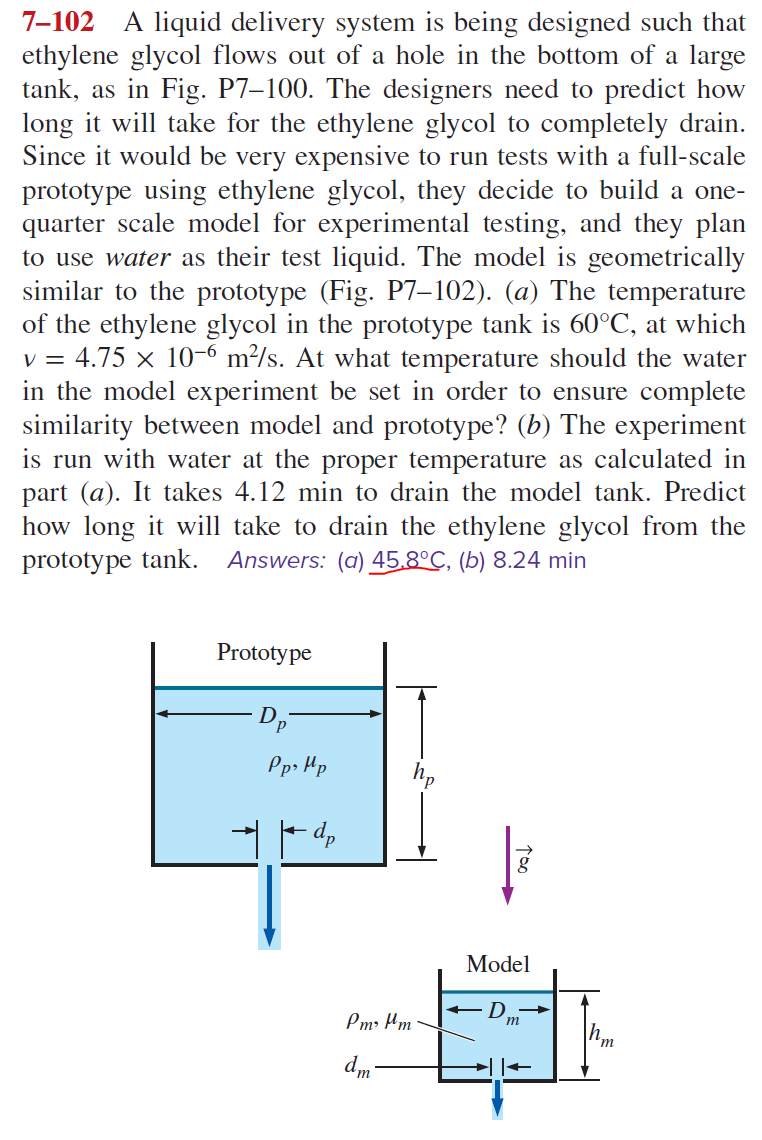
Transcribed Image Text:7-102 A liquid delivery system is being designed such that
ethylene glycol flows out of a hole in the bottom of a large
tank, as in Fig. P7-100. The designers need to predict how
long it will take for the ethylene glycol to completely drain.
Since it would be very expensive to run tests with a full-scale
prototype using ethylene glycol, they decide to build a one-
quarter scale model for experimental testing, and they plan
to use water as their test liquid. The model is geometrically
similar to the prototype (Fig. P7-102). (a) The temperature
of the ethylene glycol in the prototype tank is 60°C, at which
v = 4.75 × 10-6 m²/s. At what temperature should the water
in the model experiment be set in order to ensure complete
similarity between model and prototype? (b) The experiment
is run with water at the proper temperature as calculated in
part (a). It takes 4.12 min to drain the model tank. Predict
how long it will take to drain the ethylene glycol from the
prototype tank. Answers: (a) 45.8°C, (b) 8.24 min
Prototype
Dp
Pp, Mp
dp
Pm² μm
dm-
TOO
Model
m
'm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning