9.2 In a sample of excited mercury atoms, all of the energy levels shown in the figure below are occupied. One of the energy levels in the figure is labelled x eV. The emission spectrum of mercury shows lines at approximately 0,9 eV, 1,5 eV and 2,2 eV. Calculate x, showing all your working. 10,4 eV 9,8 eV x eV 6,7 eV 4,9 eV 0 eV
9.2 In a sample of excited mercury atoms, all of the energy levels shown in the figure below are occupied. One of the energy levels in the figure is labelled x eV. The emission spectrum of mercury shows lines at approximately 0,9 eV, 1,5 eV and 2,2 eV. Calculate x, showing all your working. 10,4 eV 9,8 eV x eV 6,7 eV 4,9 eV 0 eV
Related questions
Question
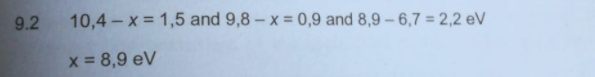
Transcribed Image Text:9.2 10,4-x = 1,5 and 9,8-x=0,9 and 8,9 - 6,7 = 2,2 eV
x = 8,9 eV
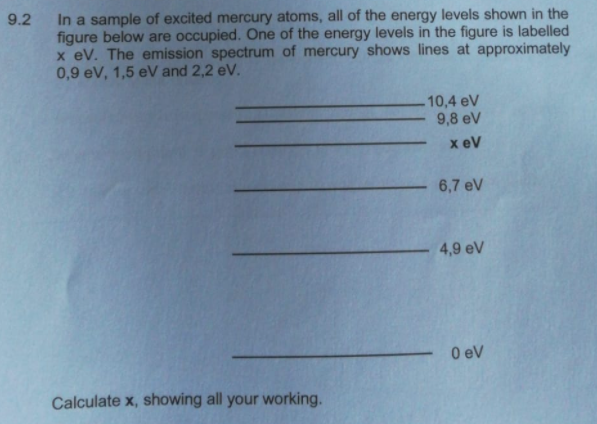
Transcribed Image Text:9.2
In a sample of excited mercury atoms, all of the energy levels shown in the
figure below are occupied. One of the energy levels in the figure is labelled
x eV. The emission spectrum of mercury shows lines at approximately
0,9 eV, 1,5 eV and 2,2 eV.
Calculate x, showing all your working.
10,4 eV
9,8 eV
x eV
6,7 eV
4,9 eV
0 eV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps
