A 0.1 M solution of acid was used to titrate 10 ml of 0.1 M solution of alkali and the following volumes of acid were recorded: 9.88 10.18 10.23 10.39 10.21 Calculate the 95% confidence limits of the mean and use them to decide whether there is any evidence of systematic error.
A 0.1 M solution of acid was used to titrate 10 ml of 0.1 M solution of alkali and the following volumes of acid were recorded: 9.88 10.18 10.23 10.39 10.21 Calculate the 95% confidence limits of the mean and use them to decide whether there is any evidence of systematic error.
Algebra: Structure And Method, Book 1
(REV)00th Edition
ISBN:9780395977224
Author:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Chapter9: Systems Of Linear Equations
Section9.5: Multiplication With The Addition-or-subtraction Method
Problem 7MRE
Related questions
Question
A 0.1 M solution of acid was used to titrate 10 ml of 0.1 M solution of alkali and the
following volumes of acid were recorded:
9.88 10.18 10.23 10.39 10.21
Calculate the 95% confidence limits of the mean and use them to decide whether
there is any evidence of systematic error.
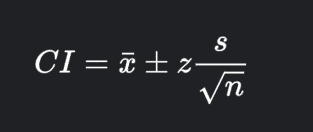
Transcribed Image Text:CI = ¤ ± z-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Elementary Algebra
Algebra
ISBN:
9780998625713
Author:
Lynn Marecek, MaryAnne Anthony-Smith
Publisher:
OpenStax - Rice University

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Elementary Algebra
Algebra
ISBN:
9780998625713
Author:
Lynn Marecek, MaryAnne Anthony-Smith
Publisher:
OpenStax - Rice University
