A 5.40 mol sample of solid A was placed in a sealed 1.00 L container and allowed to decompose into gaseous B and C. The concentration of B steadily increased until it reached 1.20 M, where it remained constant. A(s) = B(g) + C(g) Then, the container volume was doubled and equilibrium was re-established. How many moles of A remain?
A 5.40 mol sample of solid A was placed in a sealed 1.00 L container and allowed to decompose into gaseous B and C. The concentration of B steadily increased until it reached 1.20 M, where it remained constant. A(s) = B(g) + C(g) Then, the container volume was doubled and equilibrium was re-established. How many moles of A remain?
Trigonometry (MindTap Course List)
10th Edition
ISBN:9781337278461
Author:Ron Larson
Publisher:Ron Larson
Chapter6: Topics In Analytic Geometry
Section: Chapter Questions
Problem 33CT
Related questions
Question
100%
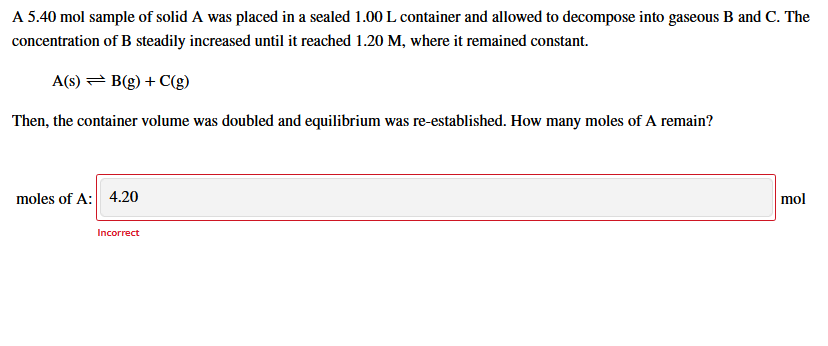
Transcribed Image Text:A 5.40 mol sample of solid A was placed in a sealed 1.00 L container and allowed to decompose into gaseous B and C. The
concentration of B steadily increased until it reached 1.20 M, where it remained constant.
A(s) = B(g) + C(g)
Then, the container volume was doubled and equilibrium was re-established. How many moles of A remain?
moles of A: 4.20
mol
Incorrect
Expert Solution
Step 1
Given:
A 5.40 mol sample of solid A was placed in a sealed 1.00 L container and allowed to decompose into gaseous B and C. The concentration of B steadily increased until it reached 1.20 M, where it remained constant.
Then, the container volume was doubled and equilibrium was re-established. How many moles of A remain?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, calculus and related others by exploring similar questions and additional content below.Recommended textbooks for you

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning