A 520.gram block of ice is at -28.0°C . What is the final temperature, how much ice is there, and how much water is there, if 1.40x10J of heat are added to it? Given that the specific heat capacity of liquid water is 4186J/(kg °C) , the specific heat capacity of ice is 2.00x10³J/(kg °C), the latent heat of vaporization of water is 2.26x10°J/kg , and the latent heat of fusion of water is 3.35x10°J/kg.
A 520.gram block of ice is at -28.0°C . What is the final temperature, how much ice is there, and how much water is there, if 1.40x10J of heat are added to it? Given that the specific heat capacity of liquid water is 4186J/(kg °C) , the specific heat capacity of ice is 2.00x10³J/(kg °C), the latent heat of vaporization of water is 2.26x10°J/kg , and the latent heat of fusion of water is 3.35x10°J/kg.
Related questions
Question
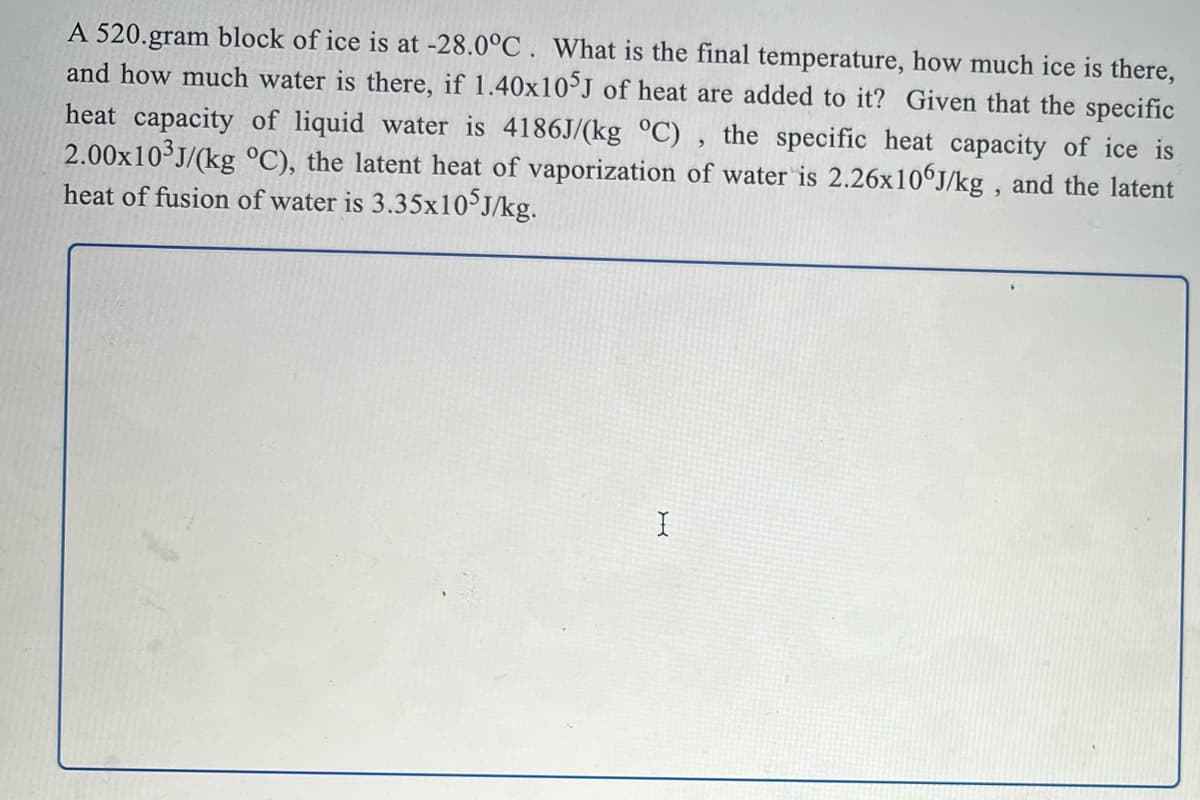
Transcribed Image Text:A 520.gram block of ice is at -28.0°C . What is the final temperature, how much ice is there,
and how much water is there, if 1.40x10J of heat are added to it? Given that the specific
heat capacity of liquid water is 4186J/(kg °C) , the specific heat capacity of ice is
2.00x10 J/(kg °C), the latent heat of vaporization of water is 2.26x106J/kg , and the latent
heat of fusion of water is 3.35×10°J/kg.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
