(a) Answer the following questions about entropy-volume relationships: (i) For a general system, use the Helmholtz free energy as an intermediary to express the derivative () in terms of a derivative of the pressure P with respect to temperature T. (ii) Assuming an ideal gas, evaluate your derivative of P, and finally integrate () to determine the volume dependence of the entropy S of the classical ideal gas. (iii) Comment on your result, and in particular on how an alternative understanding of the S(V) dependence can be achieved on the basis of spatial multiplicity considerations.
(a) Answer the following questions about entropy-volume relationships: (i) For a general system, use the Helmholtz free energy as an intermediary to express the derivative () in terms of a derivative of the pressure P with respect to temperature T. (ii) Assuming an ideal gas, evaluate your derivative of P, and finally integrate () to determine the volume dependence of the entropy S of the classical ideal gas. (iii) Comment on your result, and in particular on how an alternative understanding of the S(V) dependence can be achieved on the basis of spatial multiplicity considerations.
Related questions
Question
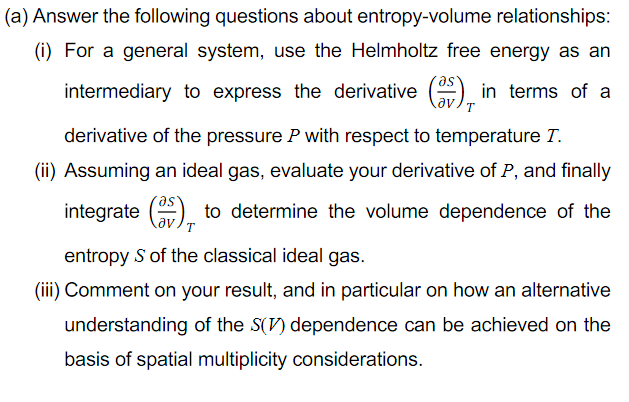
Transcribed Image Text:(a) Answer the following questions about entropy-volume relationships:
(i) For a general system, use the Helmholtz free energy as an
intermediary to express the derivative () in terms of a
T.
derivative of the pressure P with respect to temperature T.
(ii) Assuming an ideal gas, evaluate your derivative of P, and finally
integrate () to determine the volume dependence of the
av
entropy S of the classical ideal gas.
(iii) Comment on your result, and in particular on how an alternative
understanding of the S(V) dependence can be achieved on the
basis of spatial multiplicity considerations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images
