A coal-fired power plant produces a net electrical output of 800 MW. The composition the coal burned is C- 72 %, H3, 54.00% 0-6%, N-1.5%, and ash 13.5% by weight. It's heating value (HV) is 28600 A3/kg The net thermal effic 35.00% The annual capacity factor is 71.00 % A) How many metric tonnes/day of 50; are emitted if 97% of the suur in coal is converted to 50₂? 32 MONK Tres 3/12 Ca 8 How many metric RENT 0/12 day of NOx are emitted if the NOx emission rate is 265.0 ng/) of electrical input?
A coal-fired power plant produces a net electrical output of 800 MW. The composition the coal burned is C- 72 %, H3, 54.00% 0-6%, N-1.5%, and ash 13.5% by weight. It's heating value (HV) is 28600 A3/kg The net thermal effic 35.00% The annual capacity factor is 71.00 % A) How many metric tonnes/day of 50; are emitted if 97% of the suur in coal is converted to 50₂? 32 MONK Tres 3/12 Ca 8 How many metric RENT 0/12 day of NOx are emitted if the NOx emission rate is 265.0 ng/) of electrical input?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
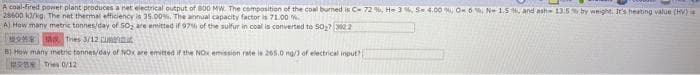
Transcribed Image Text:A coal-fired power plant produces a net electrical output of 800 MW. The composition of the coal burned is C- 72 %, H-3 %, S- 4.00 %, O 6%, N-1.5%, and ash- 13.5% by weight. It's heating value (HV) is
28600 13/kg. The net thermal efficiency is 35.00%. The annual capacity factor is 71.00%
A) How many metric tonnes/day of 50, are emitted if 97% of the sulfur in coal is converted to 50₂7 3022
MOMO Tries 3/12 Cuk
B) How many metric tonnes/day of NOx are emitted if the NOx emission rate is 265.0 ng/) of electrical input?
Tries 0/12-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The