A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 117°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 × 10° Pa on the gas. The gas is cooled until its temperature has decreased to 27° C. For the gas Cy = 11.65 J/mol · K, and the ideal gas constant R = 8.314 J/mol - K.
A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 117°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 × 10° Pa on the gas. The gas is cooled until its temperature has decreased to 27° C. For the gas Cy = 11.65 J/mol · K, and the ideal gas constant R = 8.314 J/mol - K.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter8: Natural Convection
Section: Chapter Questions
Problem 8.3P
Related questions
Question
Transcribed Image Text:Item 8
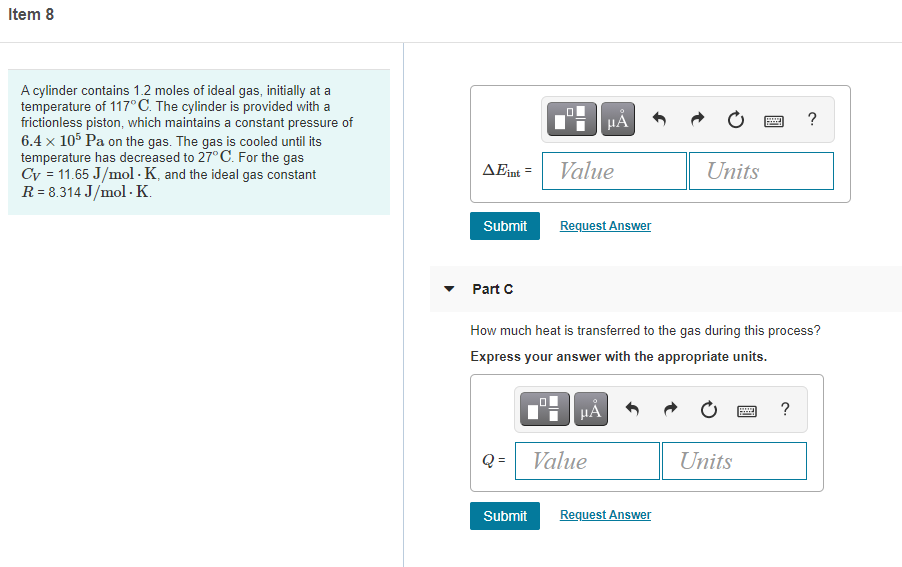
A cylinder contains 1.2 moles of ideal gas, initially at a
temperature of 117°C. The cylinder is provided with a
frictionless piston, which maintains a constant pressure of
6.4 x 105 Pa on the gas. The gas is cooled until its
temperature has decreased to 27° C. For the gas
Cy = 11.65 J/mol · K, and the ideal gas constant
R = 8.314 J/mol · K.
HA
?
ΔΕ
Value
Units
Submit
Request Answer
Part C
How much heat is transferred to the gas during this process?
Express your answer with the appropriate units.
HA
Q =
Value
Units
Submit
Request Answer
Transcribed Image Text:Item 8
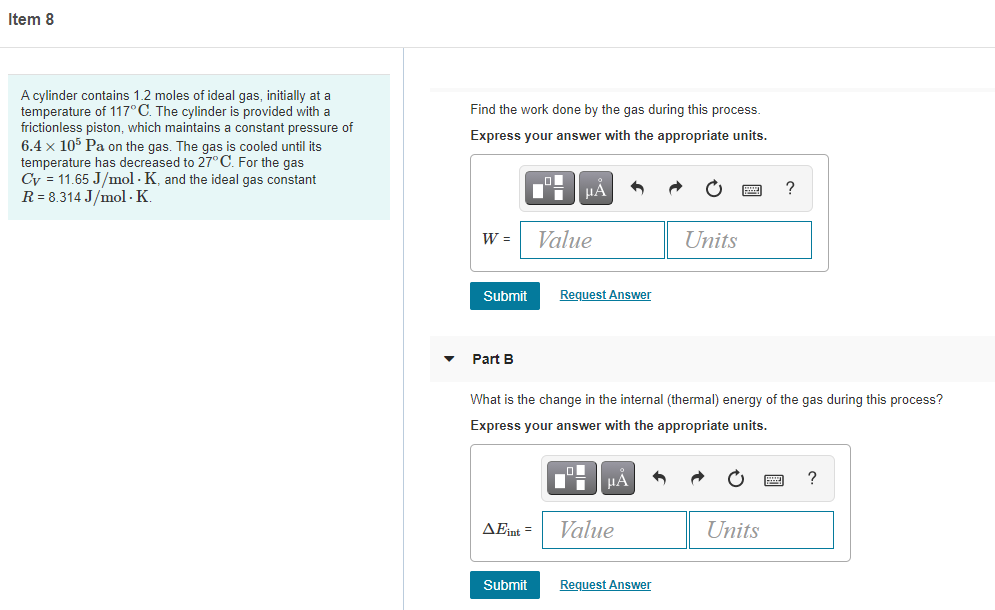
A cylinder contains 1.2 moles of ideal gas, initially at a
temperature of 117°C. The cylinder is provided with a
frictionless piston, which maintains a constant pressure of
6.4 x 105 Pa on the gas. The gas is cooled until its
temperature has decreased to 27° C. For the gas
Cy = 11.65 J/mol · K, and the ideal gas constant
R = 8.314 J/mol -K.
Find the work done by the gas during this process.
Express your answer with the appropriate units.
HẢ
?
W =
Value
Units
Submit
Request Answer
Part B
What is the change in the internal (thermal) energy of the gas during this process?
Express your answer with the appropriate units.
HA
AEint =
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning