A diatomic ideal gas expands from a volume of V₁ = 1.00 m³ to V₂ = 3.00 m² along the path shown in the figure below. The initial pressure is PA = 2.00 x 105 Pa and there are 91.5 mol of gas. P (105 Pa) 4.00 TA 3.00 2.00 B 1.00 1.00 2.00 3.00 -V (m³) (a) Calculate the work done on the gas during this process. J (b) Calculate the change in temperature of the gas. (c) Calculate the change in internal energy of the gas. (Take the molar specific heat of a diatomic gas for this process to be C₂ = — R.) J (d) How much thermal energy is transferred to the system?
A diatomic ideal gas expands from a volume of V₁ = 1.00 m³ to V₂ = 3.00 m² along the path shown in the figure below. The initial pressure is PA = 2.00 x 105 Pa and there are 91.5 mol of gas. P (105 Pa) 4.00 TA 3.00 2.00 B 1.00 1.00 2.00 3.00 -V (m³) (a) Calculate the work done on the gas during this process. J (b) Calculate the change in temperature of the gas. (c) Calculate the change in internal energy of the gas. (Take the molar specific heat of a diatomic gas for this process to be C₂ = — R.) J (d) How much thermal energy is transferred to the system?
Related questions
Question
help in this type or clear handwritting
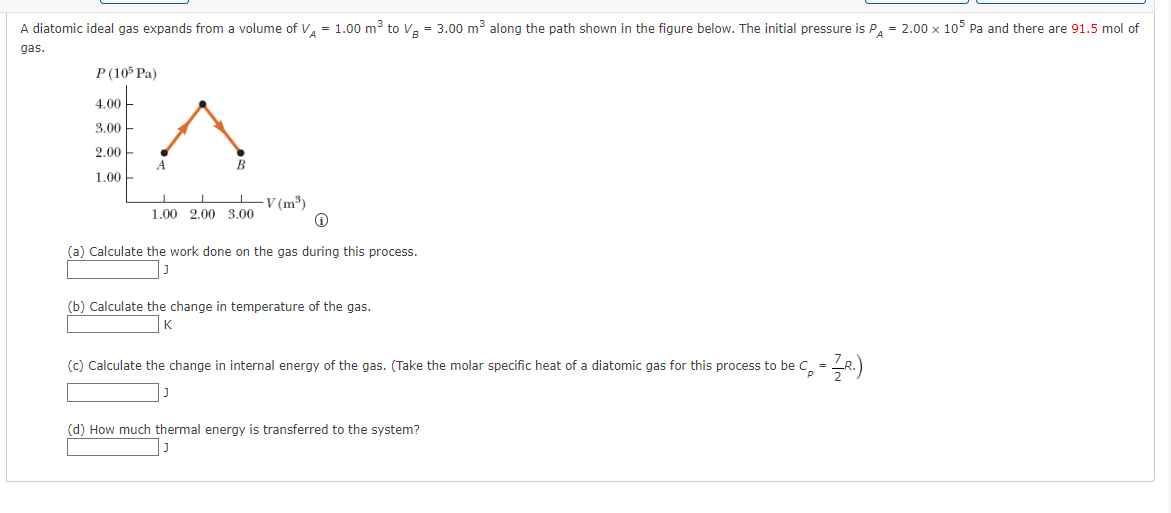
Transcribed Image Text:A diatomic ideal gas expands from a volume of V₁ = 1.00 m³ to V₂ = 3.00 m² along the path shown in the figure below. The initial pressure is PA = 2.00 x 105 Pa and there are 91.5 mol of
gas.
P (105 Pa)
4.00
TA
3.00
2.00
B
1.00
1.00 2.00 3.00
-V (m³)
(a) Calculate the work done on the gas during this process.
J
(b) Calculate the change in temperature of the gas.
(c) Calculate the change in internal energy of the gas. (Take the molar specific heat of a diatomic gas for this process to be C₂ = — R.)
J
(d) How much thermal energy is transferred to the system?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hey please submit part d also otherwise i will dislike ( i have no more question to ask separately ) understand please
Solution