A gas undergoes a process in a piston-cylinder assembly during which the pressure-specific volume relation is pv¹¹ = constant. The mass of the gas is 0.4 lb and the following data are known: p₁ = 160 lbf/in.², V₁ = 1 ft3, and p2 = 300 lbf/in.² During the process, heat transfer from the gas is 2.1 Btu. Kinetic and potential energy effects are negligible. Determine the change in specific internal energy of the gas, in Btu/lb. Au = i 31.775 Btu/lb
A gas undergoes a process in a piston-cylinder assembly during which the pressure-specific volume relation is pv¹¹ = constant. The mass of the gas is 0.4 lb and the following data are known: p₁ = 160 lbf/in.², V₁ = 1 ft3, and p2 = 300 lbf/in.² During the process, heat transfer from the gas is 2.1 Btu. Kinetic and potential energy effects are negligible. Determine the change in specific internal energy of the gas, in Btu/lb. Au = i 31.775 Btu/lb
Related questions
Question
Transcribed Image Text:* Your answer is incorrect.
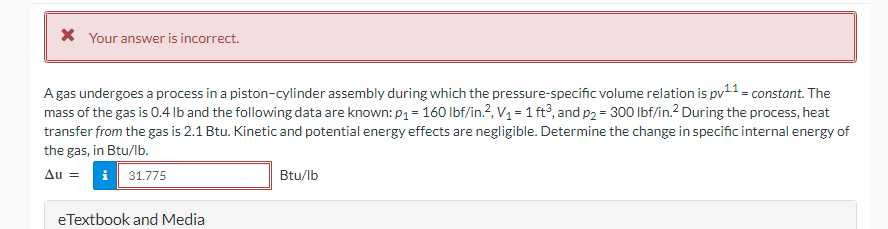
A gas undergoes a process in a piston-cylinder assembly during which the pressure-specific volume relation is pv¹¹ = constant. The
mass of the gas is 0.4 lb and the following data are known: p₁ = 160 lbf/in.², V₁ = 1 ft3, and p2 = 300 lbf/in.² During the process, heat
transfer from the gas is 2.1 Btu. Kinetic and potential energy effects are negligible. Determine the change in specific internal energy of
the gas, in Btu/lb.
Au =
31.775
eTextbook and Media
Btu/lb
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps
