A hydrogen atom in a state having a binding energy (the energy required to remove an electron) of -1.51 eV makes a transition to a state with an excitation energy (the difference between the energy of the state and that of the ground state) of 10.200 eV. (a) What is the energy of the photon emitted as a result of the transition? What are the (b) higher quantum number and (c) lower quantum number of the transition producing this emission? Use -13.60 eV as the binding energy of an electron in the ground state. (a) Number Units (b) Number Units (c) Number Units
A hydrogen atom in a state having a binding energy (the energy required to remove an electron) of -1.51 eV makes a transition to a state with an excitation energy (the difference between the energy of the state and that of the ground state) of 10.200 eV. (a) What is the energy of the photon emitted as a result of the transition? What are the (b) higher quantum number and (c) lower quantum number of the transition producing this emission? Use -13.60 eV as the binding energy of an electron in the ground state. (a) Number Units (b) Number Units (c) Number Units
Related questions
Question
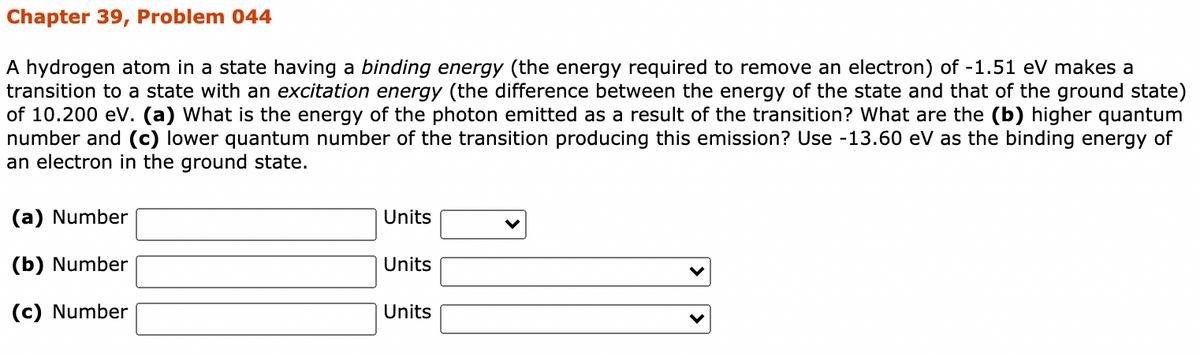
Transcribed Image Text:Chapter 39, Problem 044
A hydrogen atom in a state having a binding energy (the energy required to remove an electron) of -1.51 eV makes a
transition to a state with an excitation energy (the difference between the energy of the state and that of the ground state)
of 10.200 eV. (a) What is the energy of the photon emitted as a result of the transition? What are the (b) higher quantum
number and (c) lower quantum number of the transition producing this emission? Use -13.60 eV as the binding energy of
an electron in the ground state.
(a) Number
Units
(b) Number
Units
(c) Number
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps
