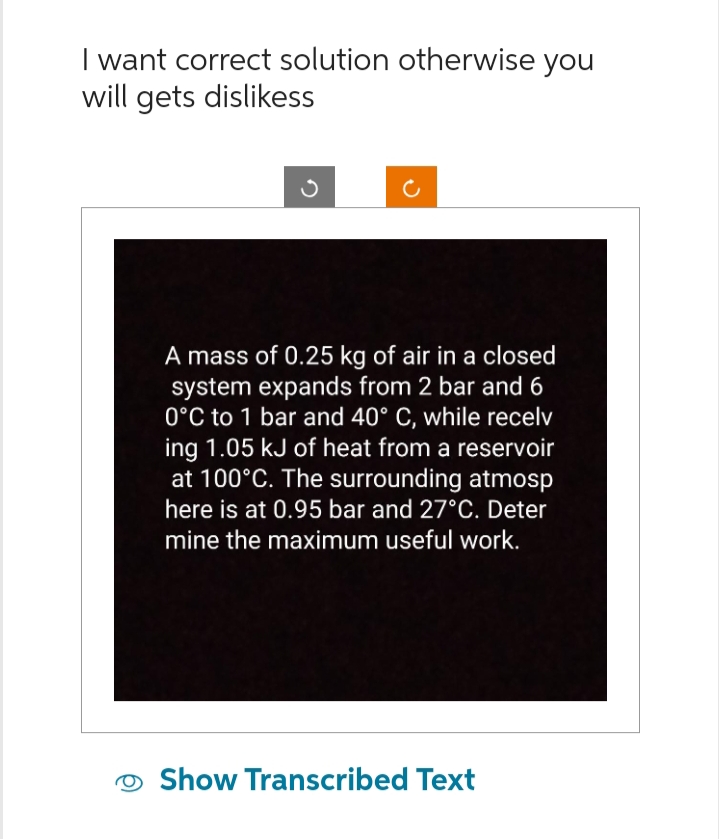
A mass of 0.25 kg of air in a closed system expands from 2 bar and 6 0°C to 1 bar and 40° C, while recelv ing 1.05 kJ of heat from a reservoir at 100°C. The surrounding atmosp here is at 0.95 bar and 27°C. Deter mine the maximum useful work.
A mass of 0.25 kg of air in a closed system expands from 2 bar and 6 0°C to 1 bar and 40° C, while recelv ing 1.05 kJ of heat from a reservoir at 100°C. The surrounding atmosp here is at 0.95 bar and 27°C. Deter mine the maximum useful work.
Related questions
Question
Subject: physics
Transcribed Image Text:I want correct solution otherwise you
will gets dislikess
c
A mass of 0.25 kg of air in a closed
system expands from 2 bar and 6
0°C to 1 bar and 40° C, while recelv
ing 1.05 kJ of heat from a reservoir
at 100°C. The surrounding atmosp
here is at 0.95 bar and 27°C. Deter
mine the maximum useful work.
Show Transcribed Text
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps
