A molecule of ozone (O3) has mass 7.97 x 10-26 kg and is travelling with speed v = 550ms in the upper atmosphere. Calculate the wavelength associated with the free particle wave (de Broglie wavelength).
Q: Prove that the momental ellipsoid at a point on the rim of a 15 -xz = constant. hemisphere is 2x² +7…
A:
Q: Is the phenomenon of radiative recombination exclusive to direct-gap semiconductors? If yes, please…
A: The phenomenon of radiative recombination exclusive to direct-gap semiconductors.Because, in the…
Q: Consider two particles: p at the origin (0,0,0) E R³ with mass M > 0, and q at the point/position…
A:
Q: A sound source with a frequency of 725 Hz moves away from a stationary observer at a rate of 15m/s…
A: When there is a relative motion between the observer and the source, the observed frequency of the…
Q: The question asks "a car with initial velocity v0 speeds up to twice its initial speed before…
A: We are given initial and final speed of car. We are also given total time of changing of speeds. We…
Q: The tension FT,n for the nth standing-wave mode are given by FT,n= 4L²f²µ/n², and thus the FT,nis…
A: Given: In this question, the given values are, The length value is, L=0.810 m. And the given…
Q: b The figure shows four circular Amperian loops (a, b, c, d) concentric with a wire whose current is…
A:
Q: Show that the moment of inertia of an elliptic area of mass M and Ma²b2 Find the radius of 4. p²…
A:
Q: QUESTION 4 For problem 36.13 calculate the intensity relative to the maximum for a point on the…
A:
Q: = 27 In Fig. 29-55, two long straight wires (shown in cross section) carry the currents i 30.0 mA…
A:
Q: ..A Rowland ring of means radius 16 cm Mas 1000 turns of wire closely wound on ferromagnetic core of…
A: We need to compute-Magnetic induction (B)=?The data given as-radius (r)=16 cm=0.16 mNo. of turns…
Q: P in a dielectric-filled parallel-plate waveguide with a separation of 9 cm between the plates, TM…
A: Given data, fTM3=6 GHz. Number of mode m = 3. Distance d = 9 cm. Speed of light c=3×108 cm/s.
Q: A bob of mass 0.320 kg is attached to one end of a light inextensible string of length 0.600 m,…
A: We are given circular motion of bob. We are given length of string. We first find radius of circle.…
Q: in the image below, it seems as though the liquid should spill out of the bowl. What is keeping it…
A: In the above case the liquid does not spill from the bowl.
Q: A transformer with more primary turns than secondary turns will produce a secondary voltage higher…
A:
Q: A current-carrying ohmic metal wire has a cross-sectional area that gradually becomes smaller from…
A: Drift velocity is the average velocity of charged particles when an external electric field is…
Q: a Find the positon of the centre of percussion of a sector of circle, axis is in the plane of the…
A:
Q: BeamDiameter When the beam exits the laser, it is approximately 30 mm in diameter. Molecules begin…
A: The intensity of the beam is the amount of light passing through per unit area, and it is also…
Q: A thin unifrom rod has one end attached to a smooth hinge anı is allowed to fall from a horizonal…
A:
Q: Waves on a string are described by the following general equation y(x, t) = A cos(kx - wt). A…
A:
Q: Derive an equation to obtain the Ricci tensor from the Einstein tenso
A: During the course of moving along geodesics in space, one can measure the Ricci tensor by measuring…
Q: A sphere, of radius a, is suspended by a fine wire from a fixed point at a distance I from its…
A: Given Data: Let us consider the given sphere of radius a,is suspended by a wire from a fixed point…
Q: : Determine (a) K.E. (b) P.E. (c) total energy and (d) binding energy of a satellite of mass 50 kg…
A: We need to compute-Kinetic energy (KE)=?Potential energy (PE)=?Total energy (TE)=?Binding energy…
Q: A heat engine operates between a high-temperature reservoir at 610 K and a low- temperature…
A: We must determine the cycle's net change in entropy. We are aware that the low temperature reservoir…
Q: A bay magnet is oscillating in a uniform magnetic field of induction 0.4×10°³7. When the frequency…
A: We need to compute-Increase in magnetic induction (B2)=?The data given as-Magnetic induction…
Q: If a magnet is dropped north down into copper tubes what directions are the induced B vectors
A: It is mentioned that magnet is dropped north down into copper tubes. We know that, magnetic field…
Q: Need help with c and d please c) How far will the ball land on the ground (x) from the point the…
A: We can answer the questions by treating the motion as a projectile motion. The detailed steps are…
Q: hanics what is the selection rules for Raman, IR and UV-Vis, the
A: Solution : The selection rule for the Raman spectroscopy is given below: for rotational raman…
Q: Find the currents in each of the resistors fis 7 T-SA D 12-2 for 7 30 72 www ww USE 42
A: We need to Find current through each resistors.
Q: Photons from the Balmer series of hydrogen transitions are sent through a double slit. What must be…
A: We are given light coming from Balmer series. We find the wavelength of light corresponding to…
Q: A 4.39 mV emf is induced in a 2000-turn pickup coil by a 200-turn field coil connected to a function…
A:
Q: What is the minimum work needed to push a 900 kg car 850 m up along a 8.0 incline? Ignore friction
A: Given: The given values are, To find the minimum work to push the car. The given mass of the car is,…
Q: Find the tension at the top if a mass of body 3 kg is performing vertical circular motion of radius…
A: We need to compute-Tension at the top (T)=?The data given as-Mass of body (m)=3 kgRadius of path…
Q: In this problem, the distance units are meters and the time units are sec- onds. Consider two…
A:
Q: The IUGS uses percentage of minerals to classify the igneous rocks. Provide the assumptions used…
A: We use ternary diagrams to find the percentage of minerals in a rock. But there are some assumptions…
Q: 8 In Fig. 29-40, two semicircular arcs have radii R₂ = 7.80 cm and R₁ = 3.15 cm, carry current i =…
A:
Q: A 10.0 kn resistor and a capacitor are connected in series, and then a 12.0V potential difference is…
A: We will answer the question using the formula for voltage across the capacitor in an RC circuit. The…
Q: Compute the flux of = a bounded below by the plane from the z-axis. + y + zk through the curved…
A:
Q: When the load L is 8.7 m from point C, the tension T in the cable has a magnitude of 8.2 kN. Express…
A: Given,a = 8.7 mb = 3 mc = 7.3 mwe know,tanθ=opposite sideadjacent sideθ=tan-1opposite sideadjacent…
Q: Changing the intensity of the incident light i. Would increasing the intensity of the incident light…
A: Given:electrode used, Aluminiumwork function of Al, ϕAl = ϕ = 4.2 eVoriginal wavelength, λ = 175 nm…
Q: A uniform solid cylinder is placed with its axis horizontal on a plane, whose inclination to the…
A:
Q: 2. For T = 300 K, calculate the pressure (in bars) at which the mean free path of a hydrogen…
A: Given: T = 300k σ = 2.3 x 10-19 m2 formula: λ = 1/(√2 x π x σ2 x N*) N* = 1/(√2 x π x σ2 x λ) N* =…
Q: What is the temperature of a gas of CO₂ molecules whose rms speed is 309 m/s?
A: The temperature of gas CO2 molecules, whose average speed is 309 m/s, must be ascertained.
Q: The emf phasor shown in the picture is rotating counterclockwise. Is the instantaneous value of the…
A: We need to determine the nature of the phasor from the given diagram The phasor makes an angle of 30…
Q: On the exam I might change the orientation of the small loops. I might also change the number of…
A: The magnetic field across a solenoid depends on the number of loops and the current flowing through…
Q: Find the positon of the centre of percussion of a sector of a circle, axis is in the plane of the…
A:
Q: QUESTION 4 For problem 33.69 find the Brewster angle in degrees if the index of refraction of…
A:
Q: Aung is placed with its axis horizontal on a plane, whose inclination to the horizon is a. Show that…
A:
Q: Suppose your favorite TV scientist has equal masses of frozen water (ice) and liquid water. If she…
A: Latent heat is the heat required to convert one gram of a substance to change its phase. Its SI unit…
Q: Suppose the total resistive force R to the motion of a car moving in a straight line is directly…
A: We are given a speed which is constant. We find the forces acting on the car. We then apply…
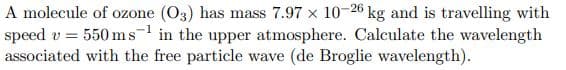
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
