A nonrelativistic electron is confined to a length of S00 pm on theaxis. What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? h=1.055 - 1034.s m9.11 10 31 1 ev-1.60 x 10-19 O 0.0038 O 0038 ev O 0.00038 v O 030
A nonrelativistic electron is confined to a length of S00 pm on theaxis. What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? h=1.055 - 1034.s m9.11 10 31 1 ev-1.60 x 10-19 O 0.0038 O 0038 ev O 0.00038 v O 030
Related questions
Question
5
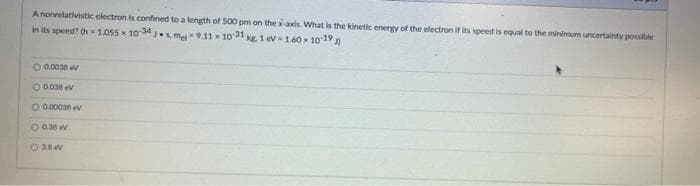
Transcribed Image Text:A nonrelativistic electron is confined to a length of S00 pm on thexaxis. What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible
in its speed? h=1.055 1034.s m9.11 x 10 31 g 1 ev-1.60 x 10-19
O 0.0038
O 0038 ev
O 0.00038
O 038 V
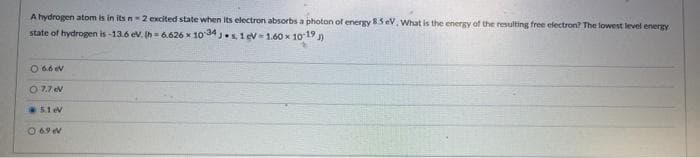
Transcribed Image Text:A hydrogen atom is in its n2 excited state when its electron absorbs a photon of energy 85 eV. What is the energy of the resulting free electron? The lowest level energy
state of hydrogen is-13.6 ev. Ih= 6.626 x 10 34 .s, 1 ev= 1.60 x 10 19 )
O 66 ev
O 7.7 ev
5.1 ev
O 69 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images
