A system consists of one mole of van der Waals gas, whose equation of state is P+ (V – b) = RT , where R is the universal gas constant. Using the information from (a) (ii), show that, for such a system, the molar volume at the critical point is given by V. = 3b. Show also that the other two parameters at the critical point are 8a and 27 Rb a Pc 27b?
A system consists of one mole of van der Waals gas, whose equation of state is P+ (V – b) = RT , where R is the universal gas constant. Using the information from (a) (ii), show that, for such a system, the molar volume at the critical point is given by V. = 3b. Show also that the other two parameters at the critical point are 8a and 27 Rb a Pc 27b?
Related questions
Question
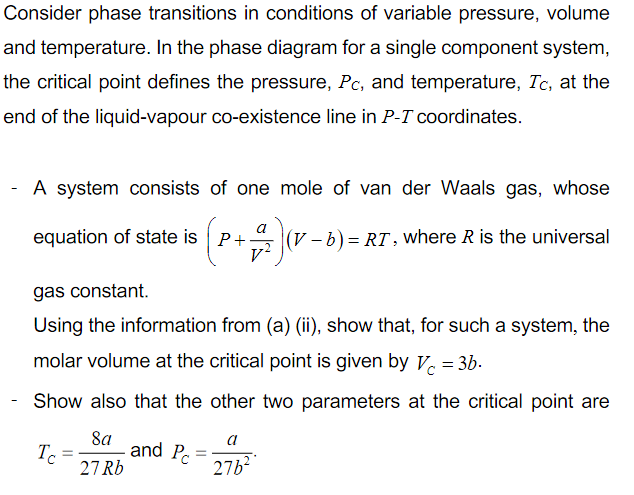
Transcribed Image Text:Consider phase transitions in conditions of variable pressure, volume
and temperature. In the phase diagram for a single component system,
the critical point defines the pressure, Pc, and temperature, Tc, at the
end of the liquid-vapour co-existence line in P-T coordinates.
A system consists of one mole of van der Waals gas, whose
equation of state is P+
(V - b) = RT , where R is the universal
gas constant.
Using the information from (a) (ii), show that, for such a system, the
molar volume at the critical point is given by V. = 3b.
Show also that the other two parameters at the critical point are
8a
and Pc
Tc
27 Rb
a
27b2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images
