A thermodynamic engine can be thought of as a cyclic process by which heat (TdS) pumped from a "hot" reservoir into a system (the engine) is converted into mechanical work (pdV) done by the engine. The most famous such example is the Carnot cycle. Another such cycle is the Otto cycle, schematically shown in the diagram below as a directed path in the volume-entropy plane. The Otto cycle is a rough approximation to the operation of a gasoline engine. Sc D SB VA VB The gas is compessed adiabatically (constant entropy) in the step A to B. Then it is heated isochorically (constant volume) in the step B to C (this step corresponds to the combustion of the gas in the gas engine). The gas is then expanded adiabatically in step C to D (this is the power stroke). Finally the gas is cooled isochorically to bring it back to its initial state; this is step D to A. Step B to C extracts heat QBc from the "hot" reservoir to heat the gas, while step D to A returns heat QDA (usually the ambient atmosphere). The difference is the work done by the engine. QAD to the "cold" reservoir If the engine efficiency is defined as ratio of the net heat converted to work, divided by the heat withdran from the hot reservoir ɛ = (QBc - QAD)/QBc %3D then assuming the gas is an ideal gas, show that the efficiency of the Otto cycle is given by, ɛ = 1- (VeV)(C, - CyyCv
A thermodynamic engine can be thought of as a cyclic process by which heat (TdS) pumped from a "hot" reservoir into a system (the engine) is converted into mechanical work (pdV) done by the engine. The most famous such example is the Carnot cycle. Another such cycle is the Otto cycle, schematically shown in the diagram below as a directed path in the volume-entropy plane. The Otto cycle is a rough approximation to the operation of a gasoline engine. Sc D SB VA VB The gas is compessed adiabatically (constant entropy) in the step A to B. Then it is heated isochorically (constant volume) in the step B to C (this step corresponds to the combustion of the gas in the gas engine). The gas is then expanded adiabatically in step C to D (this is the power stroke). Finally the gas is cooled isochorically to bring it back to its initial state; this is step D to A. Step B to C extracts heat QBc from the "hot" reservoir to heat the gas, while step D to A returns heat QDA (usually the ambient atmosphere). The difference is the work done by the engine. QAD to the "cold" reservoir If the engine efficiency is defined as ratio of the net heat converted to work, divided by the heat withdran from the hot reservoir ɛ = (QBc - QAD)/QBc %3D then assuming the gas is an ideal gas, show that the efficiency of the Otto cycle is given by, ɛ = 1- (VeV)(C, - CyyCv
Related questions
Question
100%
It's a
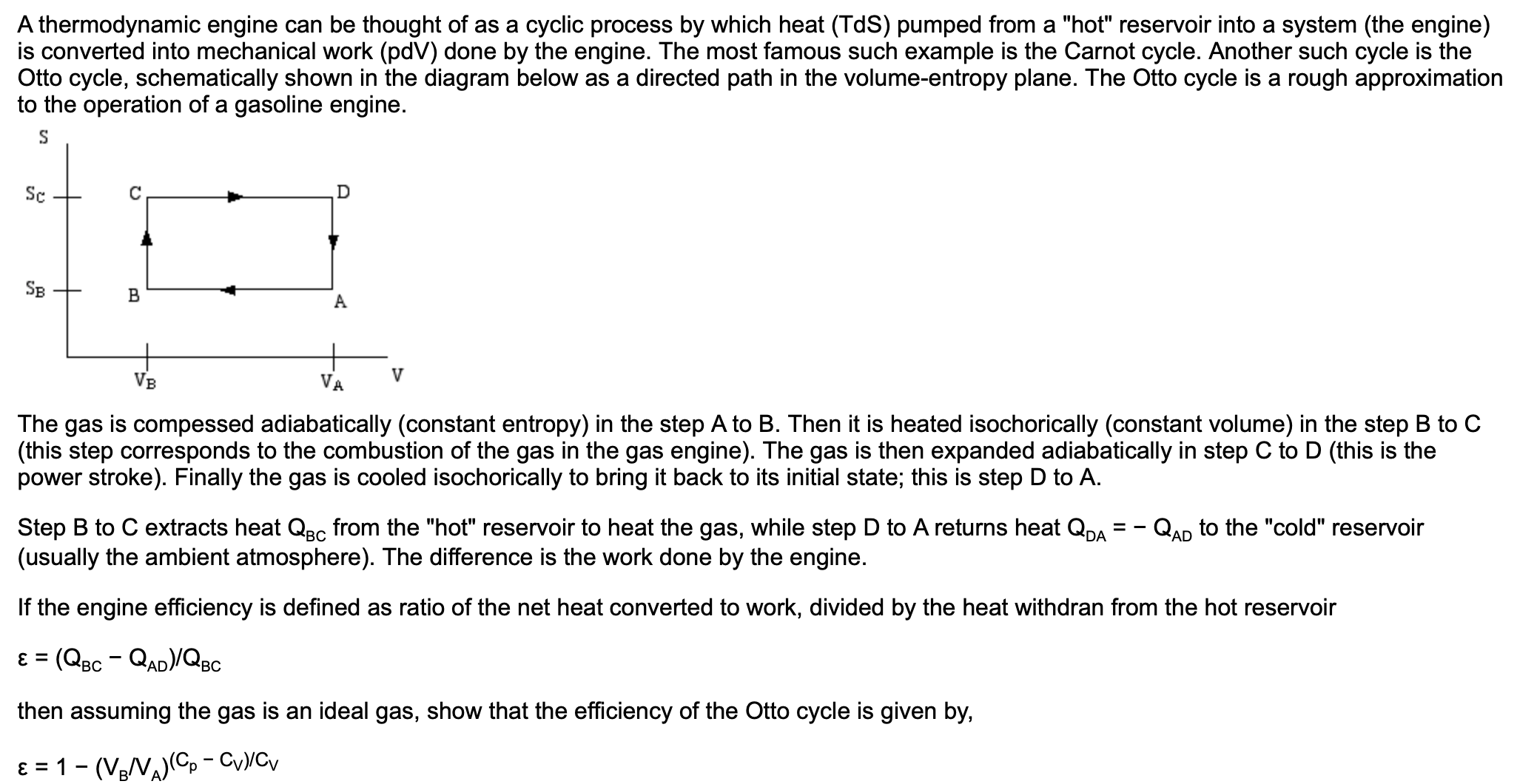
Transcribed Image Text:A thermodynamic engine can be thought of as a cyclic process by which heat (TdS) pumped from a "hot" reservoir into a system (the engine)
is converted into mechanical work (pdV) done by the engine. The most famous such example is the Carnot cycle. Another such cycle is the
Otto cycle, schematically shown in the diagram below as a directed path in the volume-entropy plane. The Otto cycle is a rough approximation
to the operation of a gasoline engine.
Sc
D
SB
VA
VB
The gas is compessed adiabatically (constant entropy) in the step A to B. Then it is heated isochorically (constant volume) in the step B to C
(this step corresponds to the combustion of the gas in the gas engine). The gas is then expanded adiabatically in step C to D (this is the
power stroke). Finally the gas is cooled isochorically to bring it back to its initial state; this is step D to A.
Step B to C extracts heat QBc from the "hot" reservoir to heat the gas, while step D to A returns heat QDA
(usually the ambient atmosphere). The difference is the work done by the engine.
QAD to the "cold" reservoir
If the engine efficiency is defined as ratio of the net heat converted to work, divided by the heat withdran from the hot reservoir
ɛ = (QBc - QAD)/QBc
%3D
then assuming the gas is an ideal gas, show that the efficiency of the Otto cycle is given by,
ɛ = 1- (VeV)(C, - CyyCv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images
