a) Using Table from no. 2, calculate the densities of the following solids: Al, Fe, Zn, and Si. b) The density of NaCl is 2.16×10³ kg/m³. Calculate the unit cell parameter (the length of the edge of a cubic cell).
a) Using Table from no. 2, calculate the densities of the following solids: Al, Fe, Zn, and Si. b) The density of NaCl is 2.16×10³ kg/m³. Calculate the unit cell parameter (the length of the edge of a cubic cell).
Related questions
Question

Transcribed Image Text:a) Using Table from no. 2, calculate the densities of the following solids: Al, Fe, Zn, and
Si. b) The density of NaCl is 2.16×10³ kg/m³. Calculate the unit cell parameter (the
length of the edge of a cubic cell).
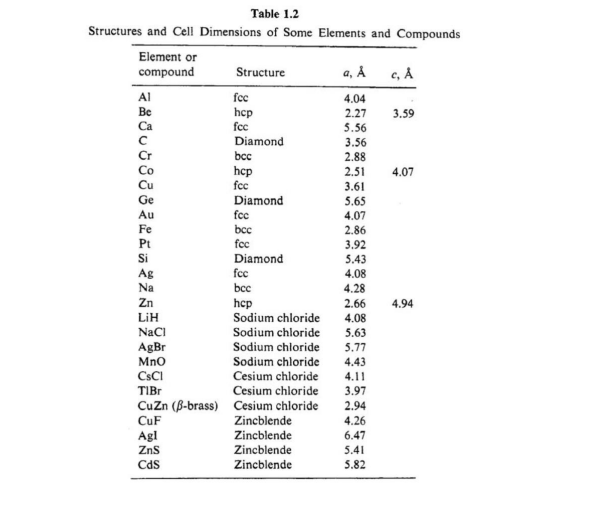
Transcribed Image Text:Table 1.2
Structures and Cell Dimensions of Some Elements and Compounds
Element or
compound
7803838 2 2 2 2 2 5
Al
Be
Pt
Si
Ag
Na
Structure
Agl
ZnS
CdS
fec
hcp
fcc
Diamond
bcc
hcp
fcc
Diamond
fec
bcc
fec
Diamond
fec
bcc
hep
LiH
NaCl
AgBr
MnO
CsCl
TIBr
Cu Zn (B-brass) Cesium chloride
CuF
Sodium chloride
Sodium chloride
Sodium chloride
Sodium chloride
Cesium chloride
Cesium chloride
Zincblende
Zincblende
Zincblende
Zincblende
a, Å
4.04
2.27
5.56
3.56
2.88
2.51
3.61
5.65
4.07
2.86
3.92
5.43
4.08
4.28
2.66
4.08
5.63
5.77
4.43
4.11
3.97
2.94
4.26
6.47
5.41
5.82
c, Å
3.59
4.07
4.94
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 11 images
