A0 and CB0= CC0= CD0 =
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Figure 1 shows the elementary reactions of ethylene oxide in series take place in a continuous constant-flow stirred tank reactor (CSTR) under isothermal conditions.
(Refer Figure 1)
The rate constant, k2=k1 + k3 and with initial CA=CA0 and CB0= CC0= CD0 =0. Given v0= 1000
L·h-1, k1= 1 min-1, k2= 2 min-1 and k3=1 min-1 and CA0= 1 mol·h-1.
Determine the expression of concentration of each components as a function of time
i.e. CA(ꞇ), CB(ꞇ), CC(ꞇ) and CD(ꞇ). ꞇ
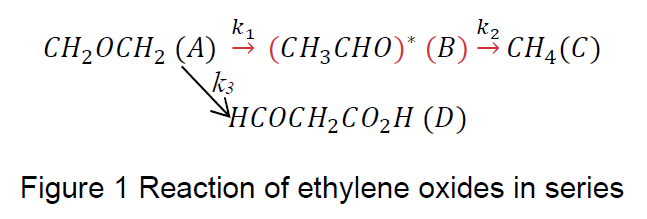
Transcribed Image Text:(CH3CHO)* (B) –CHĄ(C)
k3
HCOCH2CO2H (D)
CH20CH2 (A)
Figure 1 Reaction of ethylene oxides in series
![CONFIDENTIAL
APPENDIX 1(1)
CE/FEB 2022/CHE502
ARRHENIUS LAW
k = Ae-E/RT
r2
k2
Ег1
1
In
= In -
k1
r1
-
R [T,
T2.
IDEAL GAS CONSTANT
dm3 · atm
m3 . atm
cal
R = 0.082
= 0.082
= 1.987
= 8.314
mol · K
kmol · K
mol · K
тol - K
ENERGY BALANCE
Q - W, – FA0 )
O Cpi(T – T.) – FaoX[AH; + AG,(T – TR)] = 0
i=1
ADIABTIC TEMPERATURE
x[-AHrx(tr)] + E 0;CpiTio
Σ 0,cρTο + ΧΔCT
T =
E 0;Cpi + XAC,
EQUILIBRIUM CONVERSION
E 0;Ĉp{(T – T.)
-AHRX(TR) + 4Cp (T – TR)
X EB =
NUMERICAL METHODS
Trapezoidal rule
h
f (X) dX =
2
zF(X.) + f(X,)]
Хо
Simpson's one-third rule
h
f(X) dX =
[f (X.) + 4f (X1) + f(X2)]
3
Хо
Simpson's three-eighths rule
x3
3
F(X) ax =A[S(X,) + 3/(X,) + 3/(X2) + f(X3)I
h[f (X,) + 3f(X1) + 3f(X2) + f(X3)]
8.
Xo
© Hak Cipta Universiti Teknologi MARA
CONFIDENTIAL
CONFIDENTIAL
APPENDIX 1(2)
EH/JUN 2015/CHE584/594
Five-point quadrature formula
X4
h
"rx) ax =tr(x,) + 4f(X,) + 2f(X,) + 4f(X;) + f(X,)]
|
[f (X,) + 4f (X,)+2f(X2) + 4f(X3) + f(X4)]
3
Integrals
dx
1
= ln
1 - x
1- x
dx
1
1
(1 – x)2
1– X2
1- x1
dx
Jo (1 – x)2
1 - x
*-I _ z(x =
dx
1
= - In(1 + ɛx)
1+ EX
(1+ e)²x
(1 –x)
•* (1+ ex)² dx
= 2ɛ(1+ ɛ) In(1 – x) + e²x +
(1 – x)2
Differentiation
d
[cf (x)] = cf'(x)
dx
d
[f(x)g(x)] = f(x)g'(x) + f'(x)g(x)
dx
f (x)g'(x) + f'(x)g(x)
[S(x)] _ g(x)f'(x) – f(x)g'(x)
dx g(x)
-
[g(x)]²
d
(x")
dx
— пхп-1
© Hak Cipta Universiti Teknologi MARA
CONFIDENTIAL](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3b8dfe00-87b1-46eb-aa18-e288dbb98494%2F626c56fe-cf87-470c-a125-b54ec1a13411%2Frp5cwjd_processed.jpeg&w=3840&q=75)
Transcribed Image Text:CONFIDENTIAL
APPENDIX 1(1)
CE/FEB 2022/CHE502
ARRHENIUS LAW
k = Ae-E/RT
r2
k2
Ег1
1
In
= In -
k1
r1
-
R [T,
T2.
IDEAL GAS CONSTANT
dm3 · atm
m3 . atm
cal
R = 0.082
= 0.082
= 1.987
= 8.314
mol · K
kmol · K
mol · K
тol - K
ENERGY BALANCE
Q - W, – FA0 )
O Cpi(T – T.) – FaoX[AH; + AG,(T – TR)] = 0
i=1
ADIABTIC TEMPERATURE
x[-AHrx(tr)] + E 0;CpiTio
Σ 0,cρTο + ΧΔCT
T =
E 0;Cpi + XAC,
EQUILIBRIUM CONVERSION
E 0;Ĉp{(T – T.)
-AHRX(TR) + 4Cp (T – TR)
X EB =
NUMERICAL METHODS
Trapezoidal rule
h
f (X) dX =
2
zF(X.) + f(X,)]
Хо
Simpson's one-third rule
h
f(X) dX =
[f (X.) + 4f (X1) + f(X2)]
3
Хо
Simpson's three-eighths rule
x3
3
F(X) ax =A[S(X,) + 3/(X,) + 3/(X2) + f(X3)I
h[f (X,) + 3f(X1) + 3f(X2) + f(X3)]
8.
Xo
© Hak Cipta Universiti Teknologi MARA
CONFIDENTIAL
CONFIDENTIAL
APPENDIX 1(2)
EH/JUN 2015/CHE584/594
Five-point quadrature formula
X4
h
"rx) ax =tr(x,) + 4f(X,) + 2f(X,) + 4f(X;) + f(X,)]
|
[f (X,) + 4f (X,)+2f(X2) + 4f(X3) + f(X4)]
3
Integrals
dx
1
= ln
1 - x
1- x
dx
1
1
(1 – x)2
1– X2
1- x1
dx
Jo (1 – x)2
1 - x
*-I _ z(x =
dx
1
= - In(1 + ɛx)
1+ EX
(1+ e)²x
(1 –x)
•* (1+ ex)² dx
= 2ɛ(1+ ɛ) In(1 – x) + e²x +
(1 – x)2
Differentiation
d
[cf (x)] = cf'(x)
dx
d
[f(x)g(x)] = f(x)g'(x) + f'(x)g(x)
dx
f (x)g'(x) + f'(x)g(x)
[S(x)] _ g(x)f'(x) – f(x)g'(x)
dx g(x)
-
[g(x)]²
d
(x")
dx
— пхп-1
© Hak Cipta Universiti Teknologi MARA
CONFIDENTIAL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The