absorbed into the bloodstream, it produces carbonic acid and lowers the pH. The body compensates by producing bicarbonate, a weak base to partially neutralize the acid. The equation which models blood pH in this situation is 800 pH(x) = 6.1 + log where x is the partial pressure of carbon dioxide in arterial blood, measured in torr. Find the partial pressure of carbon dioxide in arterial blood if the pH is 7.8.
absorbed into the bloodstream, it produces carbonic acid and lowers the pH. The body compensates by producing bicarbonate, a weak base to partially neutralize the acid. The equation which models blood pH in this situation is 800 pH(x) = 6.1 + log where x is the partial pressure of carbon dioxide in arterial blood, measured in torr. Find the partial pressure of carbon dioxide in arterial blood if the pH is 7.8.
Functions and Change: A Modeling Approach to College Algebra (MindTap Course List)
6th Edition
ISBN:9781337111348
Author:Bruce Crauder, Benny Evans, Alan Noell
Publisher:Bruce Crauder, Benny Evans, Alan Noell
Chapter2: Graphical And Tabular Analysis
Section2.1: Tables And Trends
Problem 1TU: If a coffee filter is dropped, its velocity after t seconds is given by v(t)=4(10.0003t) feet per...
Related questions
Question
100%
Blood is a buffer solution. When carbon dioxide is absorbed into the bloodstream, it produces carbonic acid and lowers the pH. The body compensates by producing bicarbonate, a weak base to partially neutralize the acid. The equation which models blood pH in this situation is (cont.)
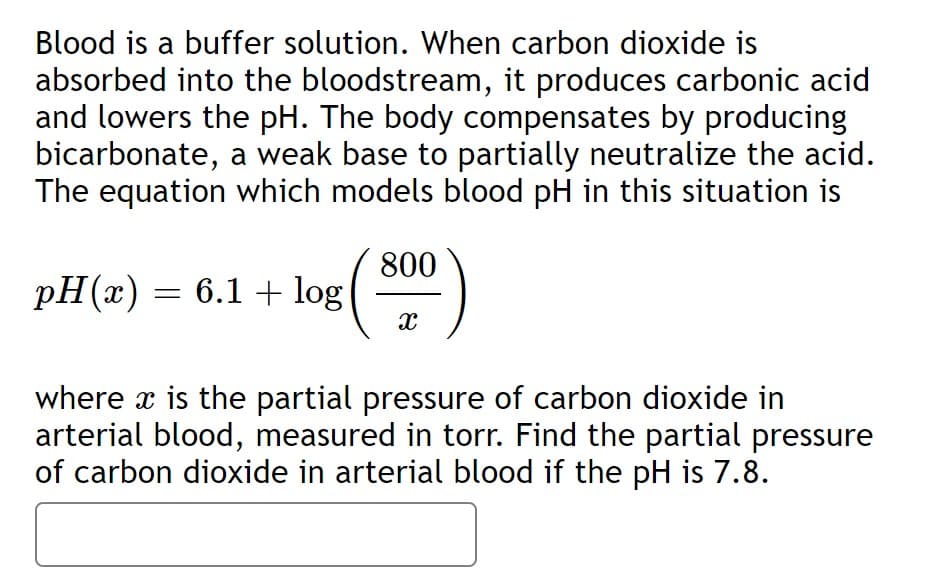
Transcribed Image Text:Blood is a buffer solution. When carbon dioxide is
absorbed into the bloodstream, it produces carbonic acid
and lowers the pH. The body compensates by producing
bicarbonate, a weak base to partially neutralize the acid.
The equation which models blood pH in this situation is
800
pH(x) = 6.1 + log
()
where x is the partial pressure of carbon dioxide in
arterial blood, measured in torr. Find the partial pressure
of carbon dioxide in arterial blood if the pH is 7.8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, calculus and related others by exploring similar questions and additional content below.Recommended textbooks for you

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage
