Acrylonitrile can be formed from propylene, ammonia, and oxygen in the reaction C3H6 + NH3 + 0₂ C3H₂N+ 3 H₂O 3 2 ->> To produce acrylonitrile, stoichiometric ratios of propylene, ammonia, and oxygen are fed into the reactor. The single pass conversion of this reaction is 30.3% of the propylene. The product of the reaction is fed into a separator where the unreacted propylene, ammonia, and oxygen are recycled back to the feed stream, and the acrylonitrile and water exit as products. 90 mol/min 91 mol C₂H./min 92 mol NH3/min 93 mol O₂/min 94 mol/min Reactor 95 mol/min 96 mol/min 97 mol C3H./min 98 mol NH3/min 99 mol O₂/min Separator 910 mol/min 12.4 kg C₂H₂N/min 911 mol H₂O/min If 12.4 kg C₂H₂N/min are desired, find the flow rate of fresh feed go, the flow rate of the recycle stream q6, and the ratio of fresh feed to recycled feed that enters the reactor.
Acrylonitrile can be formed from propylene, ammonia, and oxygen in the reaction C3H6 + NH3 + 0₂ C3H₂N+ 3 H₂O 3 2 ->> To produce acrylonitrile, stoichiometric ratios of propylene, ammonia, and oxygen are fed into the reactor. The single pass conversion of this reaction is 30.3% of the propylene. The product of the reaction is fed into a separator where the unreacted propylene, ammonia, and oxygen are recycled back to the feed stream, and the acrylonitrile and water exit as products. 90 mol/min 91 mol C₂H./min 92 mol NH3/min 93 mol O₂/min 94 mol/min Reactor 95 mol/min 96 mol/min 97 mol C3H./min 98 mol NH3/min 99 mol O₂/min Separator 910 mol/min 12.4 kg C₂H₂N/min 911 mol H₂O/min If 12.4 kg C₂H₂N/min are desired, find the flow rate of fresh feed go, the flow rate of the recycle stream q6, and the ratio of fresh feed to recycled feed that enters the reactor.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Please post steps!!
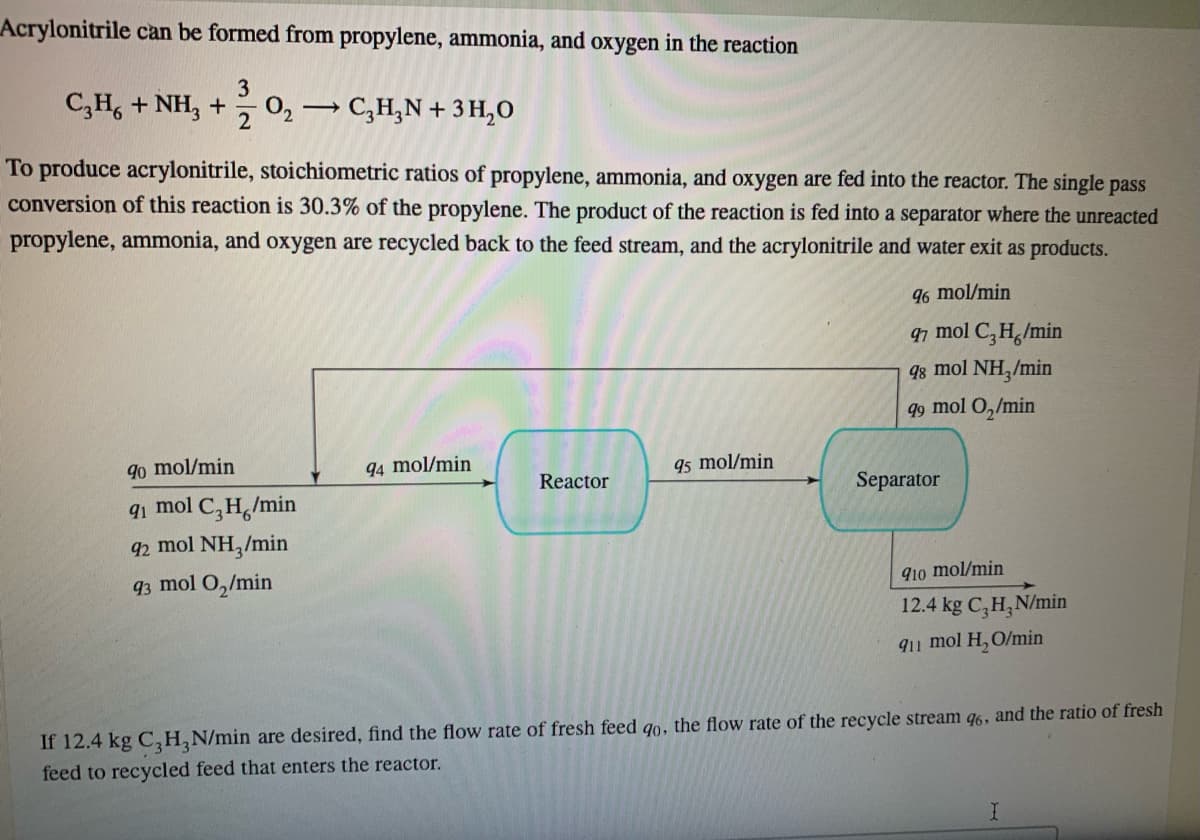
Transcribed Image Text:Acrylonitrile can be formed from propylene, ammonia, and oxygen in the reaction
3
C3H6 + NH3 + O₂ →
2
->
C3H₂N+ 3 H₂O
To produce acrylonitrile, stoichiometric ratios of propylene, ammonia, and oxygen are fed into the reactor. The single pass
conversion of this reaction is 30.3% of the propylene. The product of the reaction is fed into a separator where the unreacted
propylene, ammonia, and oxygen are recycled back to the feed stream, and the acrylonitrile and water exit as products.
90 mol/min
91 mol C3H./min
92 mol NH3/min
93 mol O₂/min
94 mol/min
Reactor
95 mol/min
96 mol/min
97 mol C₂H./min
g8 mol NH,/min
99 mol O₂/min
Separator
910 mol/min
12.4 kg C₂H₂N/min
911 mol H₂O/min
If 12.4 kg C₂H₂N/min are desired, find the flow rate of fresh feed go, the flow rate of the recycle stream 96, and the ratio of fresh
feed to recycled feed that enters the reactor.
I

Transcribed Image Text:If 12.4 kg C₂H₂N/min are desired, find the flow rate of fresh feed go, the flow rate of the recycle stream q6, and the ratio of fresh
feed to recycled feed that enters the reactor.
90=
96 =
90
96
mol/min
mol/min
min/min
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The