After removing certain quantity of water, the concentrated polymer solution is treated with hydrogen gas stored at 358K in 3.0 m internal diameter, 5cm thick spherical container made of nickel. Molar concentration of hydrogen in Ni at the inner surface is 0.09 kg mole/m³ and is equal to zero at outer surface. Mass diffusivity of hydrogen into Ni, D=1.2 x10-12 m/s Determine the molar diffusion rate of hydrogen through the walls of the container
After removing certain quantity of water, the concentrated polymer solution is treated with hydrogen gas stored at 358K in 3.0 m internal diameter, 5cm thick spherical container made of nickel. Molar concentration of hydrogen in Ni at the inner surface is 0.09 kg mole/m³ and is equal to zero at outer surface. Mass diffusivity of hydrogen into Ni, D=1.2 x10-12 m/s Determine the molar diffusion rate of hydrogen through the walls of the container
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
question in image
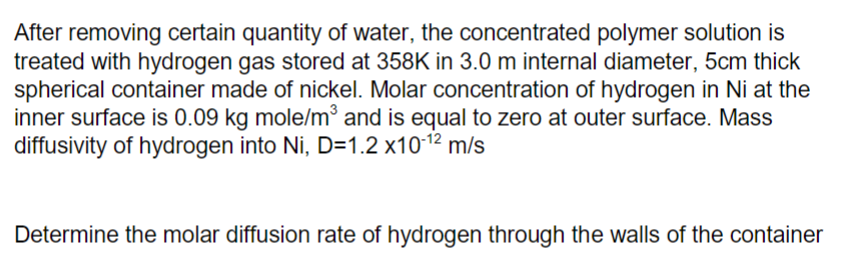
Transcribed Image Text:After removing certain quantity of water, the concentrated polymer solution is
treated with hydrogen gas stored at 358K in 3.0 m internal diameter, 5cm thick
spherical container made of nickel. Molar concentration of hydrogen in Ni at the
inner surface is 0.09 kg mole/m and is equal to zero at outer surface. Mass
diffusivity of hydrogen into Ni, D=1.2 x1012
m/s
Determine the molar diffusion rate of hydrogen through the walls of the container
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The