Air is compressed in a piston-cylinder assembly from p₁ = 25 lb/in², T₁ = 500°R, V₁ = 9 ft3 to a final volume of V₂ = 1 ft³ in a process described by pv' 1.30 = constant. Assume ideal gas behavior and neglect kinetic and potential energy effects. Using constant specific heats evaluated at T₁, determine the work and the heat transfer, in Btu.
Air is compressed in a piston-cylinder assembly from p₁ = 25 lb/in², T₁ = 500°R, V₁ = 9 ft3 to a final volume of V₂ = 1 ft³ in a process described by pv' 1.30 = constant. Assume ideal gas behavior and neglect kinetic and potential energy effects. Using constant specific heats evaluated at T₁, determine the work and the heat transfer, in Btu.
Related questions
Question
T14e please help me with my reviewer, I can’t get the value for the step 2, also kindly give me a detail solution so that I can’t I understand it thank you
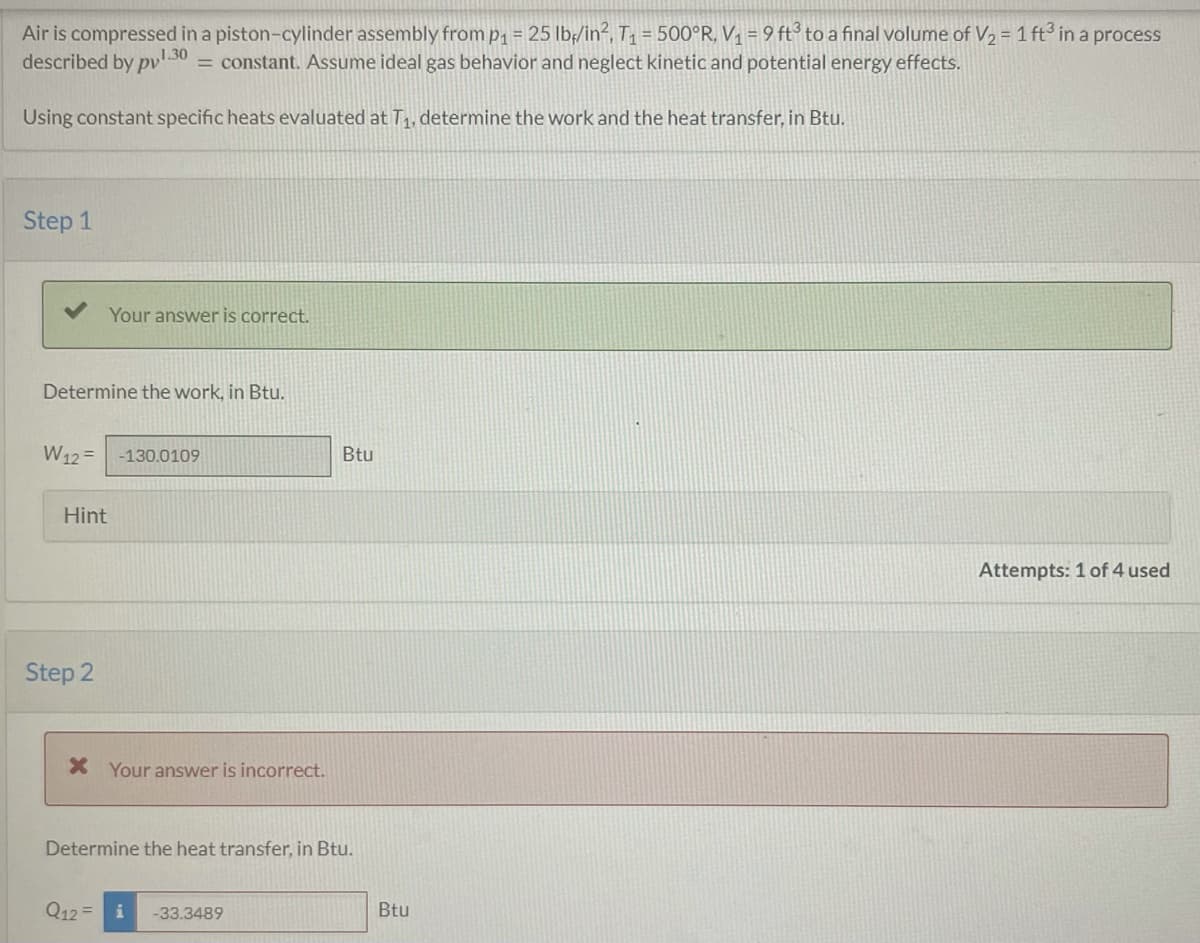
Transcribed Image Text:Air is compressed in a piston-cylinder assembly from p₁ = 25 lb/in², T₁ = 500°R, V₁ = 9 ft³ to a final volume of V₂ = 1 ft³ in a process
described by pv1.30 = constant. Assume ideal gas behavior and neglect kinetic and potential energy effects.
Using constant specific heats evaluated at T₁, determine the work and the heat transfer, in Btu.
Step 1
Determine the work, in Btu.
Your answer is correct.
W12= -130.0109
Hint
Step 2
* Your answer is incorrect.
Q12=
Determine the heat transfer, in Btu.
Btu
-33.3489
Btu
Attempts: 1 of 4 used
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
