An atom of uranium-235 (atomic mass: m(235U) cays to thorium-231 (atomic mass: m(23 Th) = 231.03630 u) 39.8 235.04392 u) de- 92 via the 90 emission of an a particle (nuclear mass: m(;a) c = 2.998 x 108 m s-1. 4.00151 u). Use
An atom of uranium-235 (atomic mass: m(235U) cays to thorium-231 (atomic mass: m(23 Th) = 231.03630 u) 39.8 235.04392 u) de- 92 via the 90 emission of an a particle (nuclear mass: m(;a) c = 2.998 x 108 m s-1. 4.00151 u). Use
Related questions
Question
Please answer parts C, D and E. Thank you in advance!
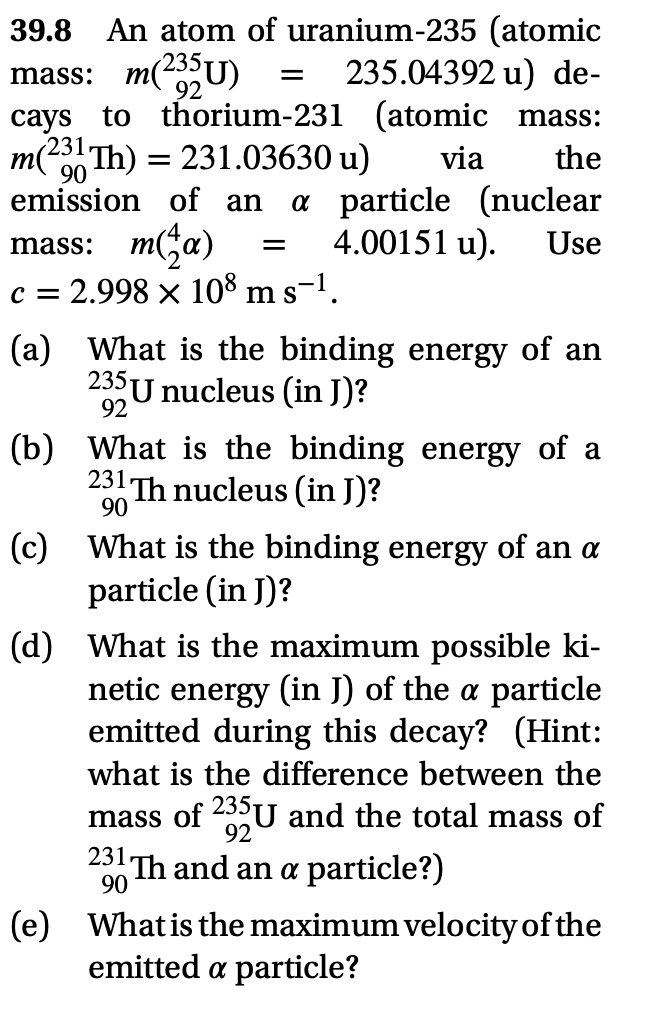
Transcribed Image Text:39.8
An atom of uranium-235 (atomic
mass: m(23U)
cays to thorium-231 (atomic mass:
231.
00 Th) = 231.03630 u)
emission of an
235.04392 u) de-
92
via
the
a particle (nuclear
4.00151 u).
m(ža)
c = 2.998 x 108 m s-1.
mass:
Use
%D
(a) What is the binding energy of an
235U nucleus (in J)?
92
(b) What is the binding energy of a
231 Th nucleus (in J)?
90
(c) What is the binding energy of an a
particle (in J)?
(d) What is the maximum possible ki-
netic energy (in J) of the a particle
emitted during this decay? (Hint:
what is the difference between the
mass of 23°U and the total mass of
92
231 Th and an a particle?)
90
(e) Whatis the maximum velocity of the
emitted a particle?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
