An electron is revolving around a proton in a circular orbit of radius r. The proton is assumed to be stationary. The total energy of this system is p? 1 e? E 2m 4TE, r where p and m denote the momentum and mass of the electron, respectively. Take the radius r to be an estimate of the uncertainty in position Ar, and the uncertainty in momentum Ap to be an estimate of p. Suppose that ArAp = ħ when the system is in the ground state. Show that the ground state energy is given by 1 me4 e 8h? E1 Give the numerical value for E, in electronvolts. Discuss if your results are consistent with Bohr's model for the hydrogen atom.
An electron is revolving around a proton in a circular orbit of radius r. The proton is assumed to be stationary. The total energy of this system is p? 1 e? E 2m 4TE, r where p and m denote the momentum and mass of the electron, respectively. Take the radius r to be an estimate of the uncertainty in position Ar, and the uncertainty in momentum Ap to be an estimate of p. Suppose that ArAp = ħ when the system is in the ground state. Show that the ground state energy is given by 1 me4 e 8h? E1 Give the numerical value for E, in electronvolts. Discuss if your results are consistent with Bohr's model for the hydrogen atom.
Related questions
Question
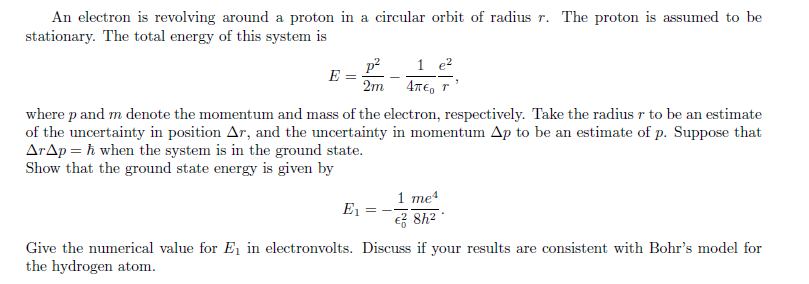
Transcribed Image Text:An electron is revolving around a proton in a circular orbit of radius r. The proton is assumed to be
stationary. The total energy of this system is
p? 1 e?
E
2m 4TE, r
where p and m denote the momentum and mass of the electron, respectively. Take the radius r to be an estimate
of the uncertainty in position Ar, and the uncertainty in momentum Ap to be an estimate of p. Suppose that
ArAp = ħ when the system is in the ground state.
Show that the ground state energy is given by
1 me4
e 8h?
E1
Give the numerical value for E, in electronvolts. Discuss if your results are consistent with Bohr's model for
the hydrogen atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images
