An ice cube is placed in an empty 80.0g aluminum cup (CAI = 0.900J /(g °C )). The ice cube (Lf= 333] /g , Ly = 2260J /g ) has an initial temperature of 0.00°C while the cup has an initial temperature of 40.0°C. The ice cube completely melts, and the melted ice and the aluminum cup reach a final equilibrium temperature of 10.0°C. No heat is lost to the outside. Calculate the mass of the ice cube (in grams).
An ice cube is placed in an empty 80.0g aluminum cup (CAI = 0.900J /(g °C )). The ice cube (Lf= 333] /g , Ly = 2260J /g ) has an initial temperature of 0.00°C while the cup has an initial temperature of 40.0°C. The ice cube completely melts, and the melted ice and the aluminum cup reach a final equilibrium temperature of 10.0°C. No heat is lost to the outside. Calculate the mass of the ice cube (in grams).
Related questions
Question
Please solve all the 3 parts, it's very very urgent
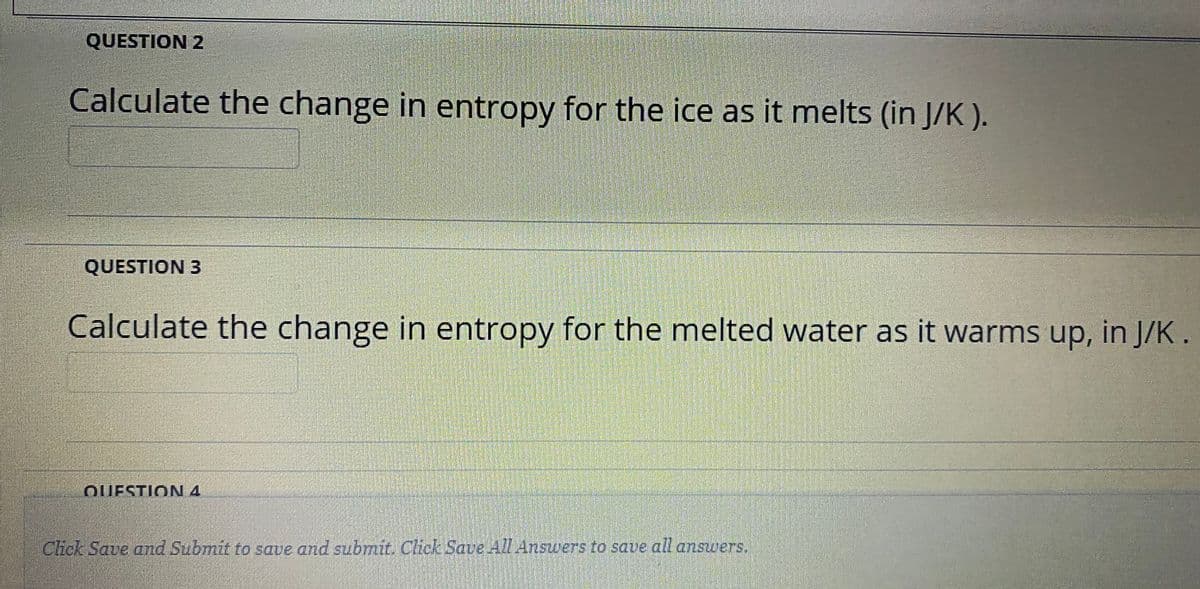
Transcribed Image Text:QUESTION 2
Calculate the change in entropy for the ice as it melts (in J/K ).
QUESTION 3
Calculate the change in entropy for the melted water as it warms up, in J/K.
QUESTION 4
CHek Save and Submit to save and submit. Chck Save 4Answers to saue all answers.
![QUESTION 1
An ice cube is placed in an empty 80.0g aluminum cup (CAI = 0.900J /(g °C )).
The ice cube (Lf = 333] /g, Ly = 2260J /g ) has an initial temperature of 0.00°C
while the cup has an initial temperature of 40.0°C. The ice cube completely
melts, and the melted ice and the aluminum cup reach a final equilibrium
temperature of 10.0°C . No heat is lost to the outside. Calculate the mass of the
ice cube (in grams).
%3D](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe8123c27-dac7-4b13-9259-036cef86f3d6%2F8c8de37b-343a-4d00-bdce-839f340b534d%2Fbl3o3wd_processed.jpeg&w=3840&q=75)
Transcribed Image Text:QUESTION 1
An ice cube is placed in an empty 80.0g aluminum cup (CAI = 0.900J /(g °C )).
The ice cube (Lf = 333] /g, Ly = 2260J /g ) has an initial temperature of 0.00°C
while the cup has an initial temperature of 40.0°C. The ice cube completely
melts, and the melted ice and the aluminum cup reach a final equilibrium
temperature of 10.0°C . No heat is lost to the outside. Calculate the mass of the
ice cube (in grams).
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 12 images
