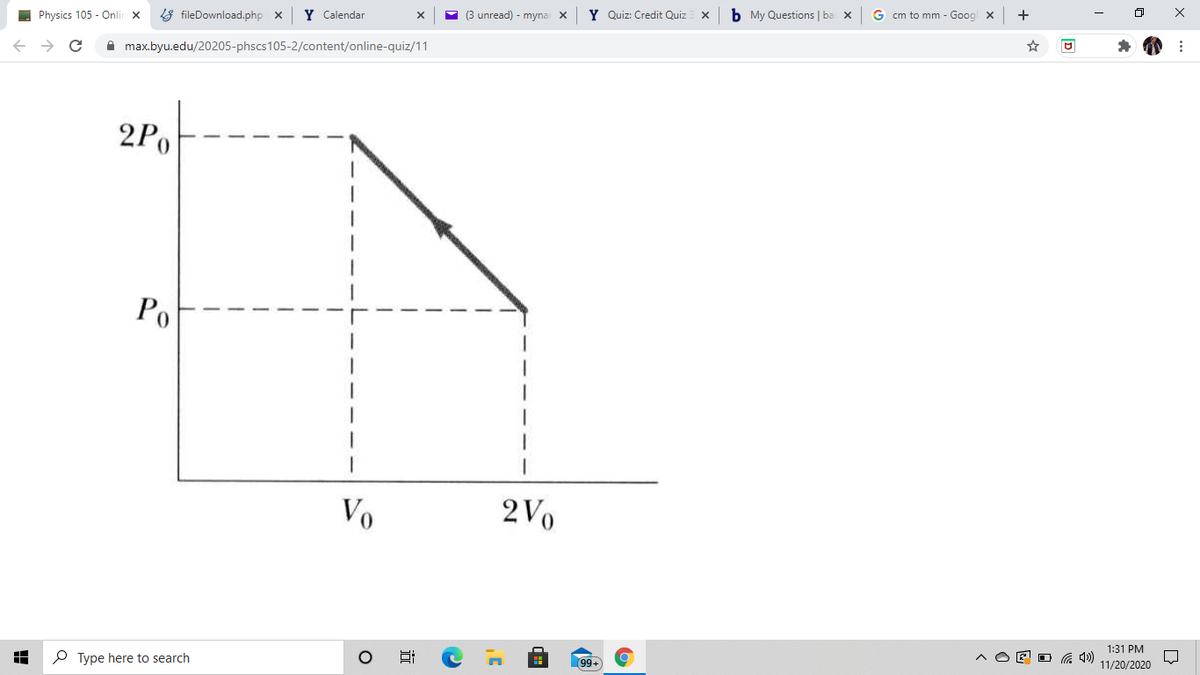
An ideal gas undergoes the thermodynamic process shown in the ??PV diagram in the figure. Determine whether each of the values (a) Δ?ΔU, (b) ?W, (c) ?Q for the gas is positive, negative, or zero. (Note that ?W is the work done ??on the gas.) Hint: First use the ideal gas law to find the initial and final temperatures in terms of ?0P0 and ?0V0 and determine if the final temperature is greater than, less than, or equal to the initial temperature.
An ideal gas undergoes the thermodynamic process shown in the ??PV diagram in the figure. Determine whether each of the values (a) Δ?ΔU, (b) ?W, (c) ?Q for the gas is positive, negative, or zero. (Note that ?W is the work done ??on the gas.) Hint: First use the ideal gas law to find the initial and final temperatures in terms of ?0P0 and ?0V0 and determine if the final temperature is greater than, less than, or equal to the initial temperature.
Related questions
Question
An ideal gas undergoes the
(a)
ΔU
A.+
B.-
C.0
(b)
W
A.+
B.-
C.0
(c)
Q
A.+
B.-
C.0
Transcribed Image Text:- Physics 105 - Onlir x
8 fileDownload.php x
Y Calendar
(3 unread) - myna X
Y Quiz: Credit Quiz
b My Questions | ba x
G cm to mm - Googl X
+
A max.byu.edu/20205-phscs105-2/content/online-quiz/11
2Po
Po
Vo
2Vo
1:31 PM
P Type here to search
99+
E O A 4)
11/20/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
