As part of a radiotherapy course a patient is injected with a sodium phosphate solution containing the radioactive isotope P, which decays via B-emission with a half-life of 14.3 days. (a) Write a balanced equation for the beta-minus decay of phosphorus-32. (b) Calculate the energy released in the decay process of phosphorus-32 in MeV units. Note that the mass of the products in this reaction is essentially the mass of the product ion plus the beta particle, i.e., the mass of the neutral atom that has the product ion as its nucleus. The relevant atomic masses are: Phosphorus-32: 31.973908 u Product atom: 31.972071 u (c) Half of the injected solution is absorbed by a 12 g tumor in the patient's body and a total of 7.5 J of energy is deposited in the tumor. Calculate the number of radioactive decay events that collectively emit this amount of energy into the tumor. (d) The radioactive phosphorus is mostly gone from the body after 24 hours due to natural processes. Use this timescale to calculate the average activity associated with this treatment, in Bq units. (e) What is the dose equivalent (in Sv and rem) that the tumor receives?
As part of a radiotherapy course a patient is injected with a sodium phosphate solution containing the radioactive isotope P, which decays via B-emission with a half-life of 14.3 days. (a) Write a balanced equation for the beta-minus decay of phosphorus-32. (b) Calculate the energy released in the decay process of phosphorus-32 in MeV units. Note that the mass of the products in this reaction is essentially the mass of the product ion plus the beta particle, i.e., the mass of the neutral atom that has the product ion as its nucleus. The relevant atomic masses are: Phosphorus-32: 31.973908 u Product atom: 31.972071 u (c) Half of the injected solution is absorbed by a 12 g tumor in the patient's body and a total of 7.5 J of energy is deposited in the tumor. Calculate the number of radioactive decay events that collectively emit this amount of energy into the tumor. (d) The radioactive phosphorus is mostly gone from the body after 24 hours due to natural processes. Use this timescale to calculate the average activity associated with this treatment, in Bq units. (e) What is the dose equivalent (in Sv and rem) that the tumor receives?
Related questions
Question
100%
Please answer questions c, d, and e. Thank you!
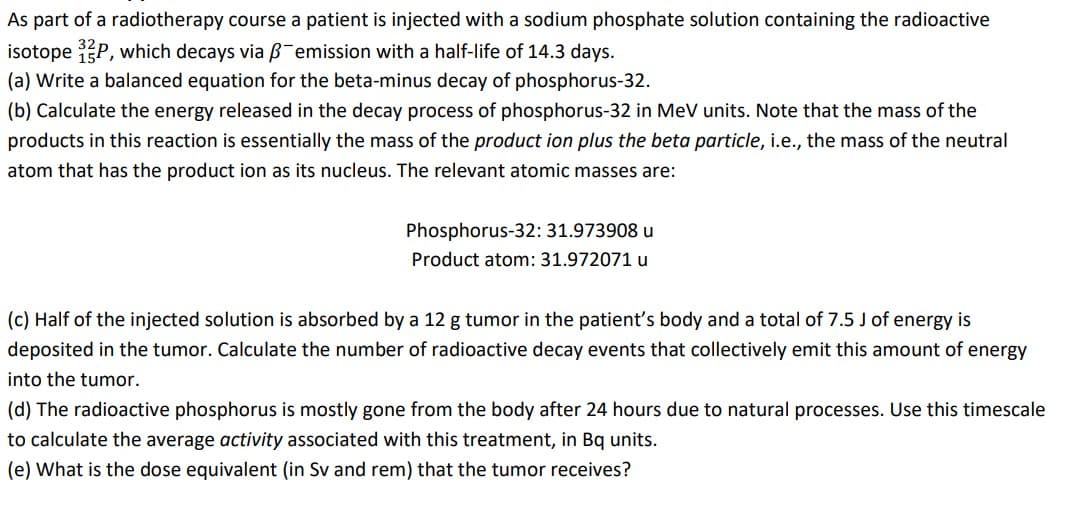
Transcribed Image Text:As part of a radiotherapy course a patient is injected with a sodium phosphate solution containing the radioactive
isotope P, which decays via B-emission with a half-life of 14.3 days.
(a) Write a balanced equation for the beta-minus decay of phosphorus-32.
(b) Calculate the energy released in the decay process of phosphorus-32 in MeV units. Note that the mass of the
products in this reaction is essentially the mass of the product ion plus the beta particle, i.e., the mass of the neutral
atom that has the product ion as its nucleus. The relevant atomic masses are:
Phosphorus-32: 31.973908 u
Product atom: 31.972071 u
(c) Half of the injected solution is absorbed by a 12 g tumor in the patient's body and a total of 7.5 J of energy is
deposited in the tumor. Calculate the number of radioactive decay events that collectively emit this amount of energy
into the tumor.
(d) The radioactive phosphorus is mostly gone from the body after 24 hours due to natural processes. Use this timescale
to calculate the average activity associated with this treatment, in Bq units.
(e) What is the dose equivalent (in Sv and rem) that the tumor receives?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
