Assuming that the vibrations of a 35C12 molecule are equivalent to those of a harmonic oscillator with a force constant k = 329 N m¯1, what is the zero-point energy of vibration of this molecule? The mass of a 3$Cl atom is 34.9688 u. How many vibrational quanta are necessary to absorb light in the visible range of the electromagnetic spectrum? What is the zero-point energy of the 37C12 molecule?
Assuming that the vibrations of a 35C12 molecule are equivalent to those of a harmonic oscillator with a force constant k = 329 N m¯1, what is the zero-point energy of vibration of this molecule? The mass of a 3$Cl atom is 34.9688 u. How many vibrational quanta are necessary to absorb light in the visible range of the electromagnetic spectrum? What is the zero-point energy of the 37C12 molecule?
Related questions
Question
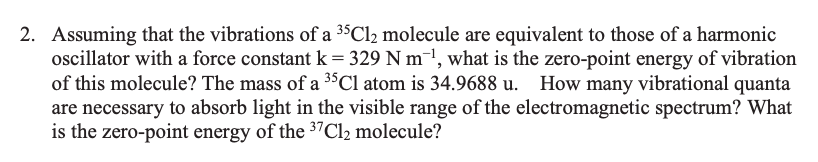
Transcribed Image Text:2. Assuming that the vibrations of a 35C12 molecule are equivalent to those of a harmonic
oscillator with a force constant k = 329 N m¯1, what is the zero-point energy of vibration
of this molecule? The mass of a 3$Cl atom is 34.9688 u. How many vibrational quanta
are necessary to absorb light in the visible range of the electromagnetic spectrum? What
is the zero-point energy of the 37C12 molecule?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps
