b) Due to hydraulic shock, the pipe ruptured resulting in release of liquid ammonia into the atmosphere. Liquid ammonia immediately flashes in the surrounding at ambient temperature, Tamb = 298 K and atmospheric pressure, Patm = 101325 Nm². Accordingly, i) starting from first principles, and stating any assumptions made, show that the mass flow rate of liquid ammonia escaping through the ruptured pipe upon failure is given by: 2(Pf - Patm) Qm=pArCou² + (1) Ρ Where: Qm = the mass flow rate, kgs¨¹ p = the fluid density, kgm³ A₁ = the pipe rupture area, m² = Co discharge coefficient u = fluid velocity inside the pipe, ms¨¹ Pf = fluid pressure inside the pipe, Nm²² Patm atmospheric pressure, Nm²
b) Due to hydraulic shock, the pipe ruptured resulting in release of liquid ammonia into the atmosphere. Liquid ammonia immediately flashes in the surrounding at ambient temperature, Tamb = 298 K and atmospheric pressure, Patm = 101325 Nm². Accordingly, i) starting from first principles, and stating any assumptions made, show that the mass flow rate of liquid ammonia escaping through the ruptured pipe upon failure is given by: 2(Pf - Patm) Qm=pArCou² + (1) Ρ Where: Qm = the mass flow rate, kgs¨¹ p = the fluid density, kgm³ A₁ = the pipe rupture area, m² = Co discharge coefficient u = fluid velocity inside the pipe, ms¨¹ Pf = fluid pressure inside the pipe, Nm²² Patm atmospheric pressure, Nm²
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
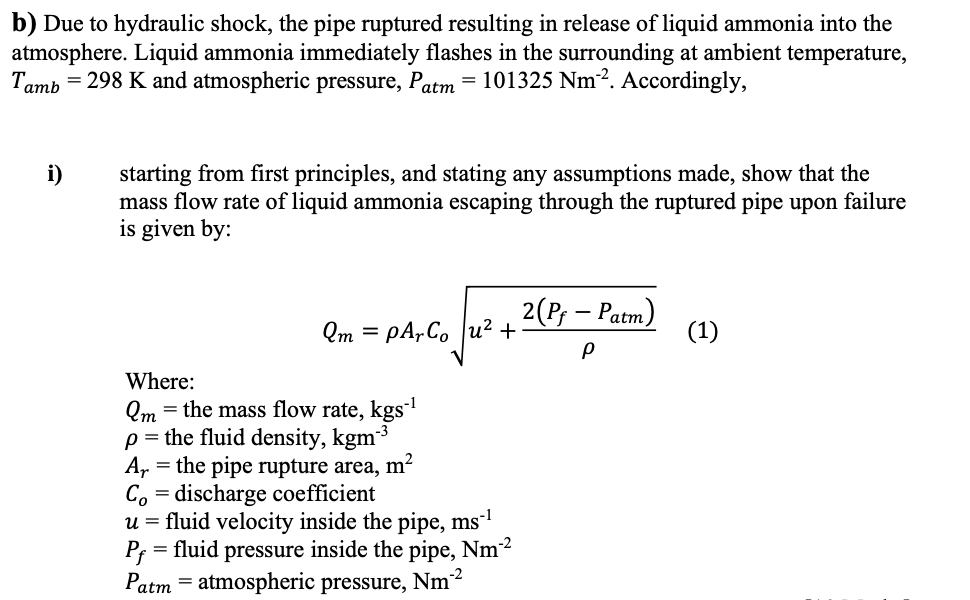
Transcribed Image Text:b) Due to hydraulic shock, the pipe ruptured resulting in release of liquid ammonia into the
atmosphere. Liquid ammonia immediately flashes in the surrounding at ambient temperature,
Tamb = 298 K and atmospheric pressure, Patm
= 101325 Nm². Accordingly,
starting from first principles, and stating any assumptions made, show that the
mass flow rate of liquid ammonia escaping through the ruptured pipe upon failure
is given by:
i)
2(P, — Расm)
(1)
Qm = pA,Co |u²
+
Where:
Qm = the mass flow rate, kgs
p = the fluid density, kgm3
A, = the pipe rupture area, m?
Co = discharge coefficient
u = fluid velocity inside the pipe, ms-1
= fluid pressure inside the pipe, Nm2
P;
Patm = atmospheric pressure, Nm³
n-2
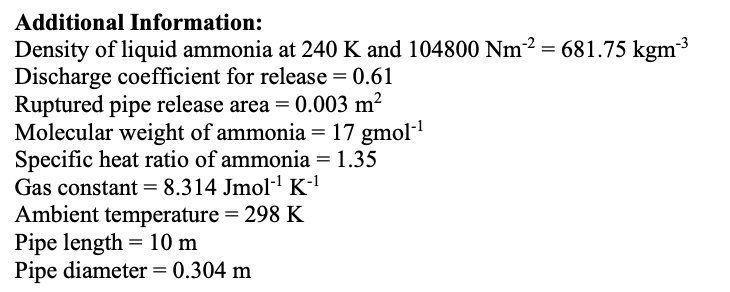
Transcribed Image Text:Additional Information:
Density of liquid ammonia at 240 K and 104800 Nm2 = 681.75 kgm3
Discharge coefficient for release = 0.61
Ruptured pipe release area = 0.003 m2
Molecular weight of ammonia = 17 gmol-1
Specific heat ratio of ammonia = 1.35
Gas constant= 8.314 Jmol-' K-'
Ambient temperature = 298 K
Pipe length = 10 m
Pipe diameter = 0.304 m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 25 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The