batch reactor.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Answers xa=0.87
CA=0.03M
Transcribed Image Text:1:55 AM Thu 6 Apr
<
Problem Shee.
20
*
Coutant
thaybut
Th
Problem Shee..
x
Untitled Noteb.
REACTION ENG
·KA = KC"
X
In
CAPE 1020 En..
T
CAPE 1020 En...
CAPE1010 Pra..
=
□ 1+9
11
к са св
RA-
CAPE1010 Lecture Handout - Reaction and Biochemical Engineering 22/23
44%
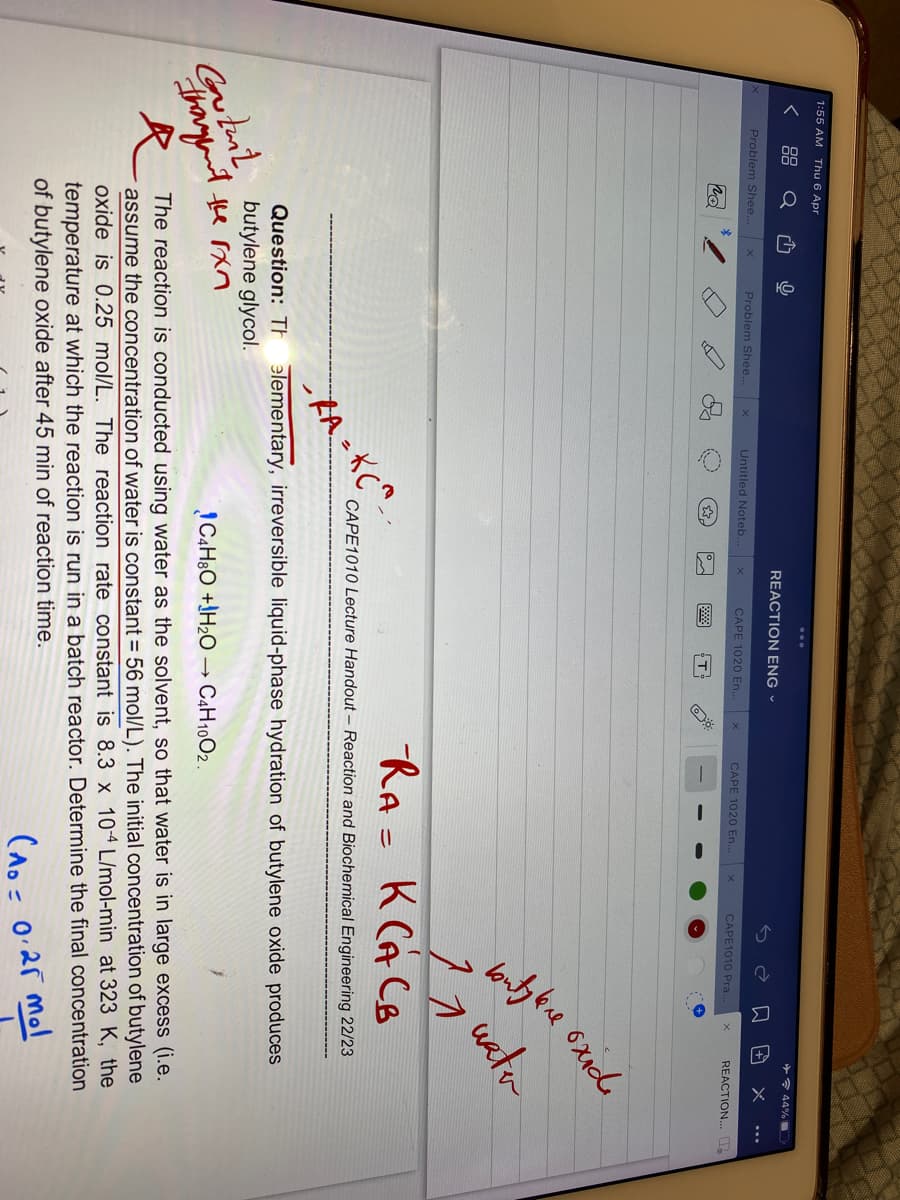
Question: The elementary, irreversible liquid-phase hydration of butylene oxide produces
butylene glycol.
the rxn
x
REACTION...
butylene oxide
water
***
C4H8O+H₂O → C4H10O2.
The reaction is conducted using water as the solvent, so that water is in large excess (i.e.
assume the concentration of water is constant = 56 mol/L). The initial concentration of butylene
oxide is 0.25 mol/L. The reaction rate constant is 8.3 x 104 L/mol-min at 323 K, the
temperature at which the reaction is run in a batch reactor. Determine the final concentration
of butylene oxide after 45 min of reaction time.
(A0 = 0.25 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The