Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene (2) system at 45°C are A12 = 0.124 and A2 = 0.523. Use these with the Wilson equation to predict liquid-phase activity coefficients over the composition range and compare them, in a plot like Figure 2.12, with the experimental results [Austral. J. Chem., 7, 264 (1954)]: In yi In y2 0.0374 2.0937 0.0220 0.0972 1.6153 0.0519 0.3141 0.7090 0.2599 0.5199 0.3136 0.5392 0.7087 0.1079 0.8645 0.9193 0.0002 1.3177 0.9591 -0.0077 1.3999
Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene (2) system at 45°C are A12 = 0.124 and A2 = 0.523. Use these with the Wilson equation to predict liquid-phase activity coefficients over the composition range and compare them, in a plot like Figure 2.12, with the experimental results [Austral. J. Chem., 7, 264 (1954)]: In yi In y2 0.0374 2.0937 0.0220 0.0972 1.6153 0.0519 0.3141 0.7090 0.2599 0.5199 0.3136 0.5392 0.7087 0.1079 0.8645 0.9193 0.0002 1.3177 0.9591 -0.0077 1.3999
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
![Benzene can break the ethanol/water azeotrope to produce
nearly pure ethanol. Wilson constants for the ethanol (1)/benzene
(2) system at 45°C are A12 = 0.124 and A21 = 0.523. Use these
with the Wilson equation to predict liquid-phase activity coefficients
over the composition range and compare them, in a plot like Figure
2.12, with the experimental results [Austral. J. Chem., 7, 264
(1954)]:
In yi
In y2
0.0374
2.0937
0.0220
0.0972
1.6153
0.0519
0.3141
0.7090
0.2599
0.5199
0.3136
0.5392
0.7087
0.1079
0.8645
0.9193
0.0002
1.3177
0.9591
-0.0077
1.3999](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb271c5e6-be73-43fb-9513-87909eee469c%2F1c67f688-41f2-4201-bf9b-097cfb2c70a5%2Fiuguir7_processed.png&w=3840&q=75)
Transcribed Image Text:Benzene can break the ethanol/water azeotrope to produce
nearly pure ethanol. Wilson constants for the ethanol (1)/benzene
(2) system at 45°C are A12 = 0.124 and A21 = 0.523. Use these
with the Wilson equation to predict liquid-phase activity coefficients
over the composition range and compare them, in a plot like Figure
2.12, with the experimental results [Austral. J. Chem., 7, 264
(1954)]:
In yi
In y2
0.0374
2.0937
0.0220
0.0972
1.6153
0.0519
0.3141
0.7090
0.2599
0.5199
0.3136
0.5392
0.7087
0.1079
0.8645
0.9193
0.0002
1.3177
0.9591
-0.0077
1.3999
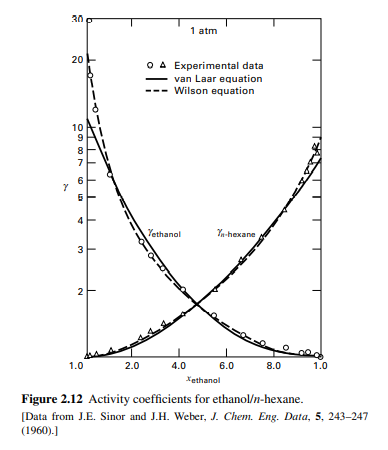
Transcribed Image Text:30
1 atm
20
O A Experimental data
van Laar equation
--- Wilson equation
10
9
8
Yethanal
Yn-hexane
3
2
1.0
2.0
4.0
6.0
8.0
1.0
Xethanol
Figure 2.12 Activity coefficients for ethanol/n-hexane.
[Data from J.E. Sinor and J.H. Weber, J. Chem. Eng. Data, 5, 243-247
(1960).)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The