Biol 190L Conversion & Concentration Practice Problems 1. Complete the metric system conversions below (use scientific notation). ug (micrograms) ug (micrograms) 1 g = 1 kg = mg 10 g = 10 kg = mg 100 g = Hg (micrograms) 100 kg = mg 1 µg (microgram) = 1 ng = g 10 µg (micrograms) = 10 ng = 100 µg (micrograms) = 100 ng = 2. Solve the following concentration problems and show your work. • How many grams of NaCl are required to prepare 140 ml of a 15% solution? How many mg of CgH1,06are required to prepare 14 ml of a 0.15% solution? 3. Solve the following concentration problems and show your work. • How many grams of NaCl are required to prepare 400 mL of a 1.50 M solution? There are 540 g of CH,,0, dissolved in 4 liters of solution. What is the molarity of this solution?
Biol 190L Conversion & Concentration Practice Problems 1. Complete the metric system conversions below (use scientific notation). ug (micrograms) ug (micrograms) 1 g = 1 kg = mg 10 g = 10 kg = mg 100 g = Hg (micrograms) 100 kg = mg 1 µg (microgram) = 1 ng = g 10 µg (micrograms) = 10 ng = 100 µg (micrograms) = 100 ng = 2. Solve the following concentration problems and show your work. • How many grams of NaCl are required to prepare 140 ml of a 15% solution? How many mg of CgH1,06are required to prepare 14 ml of a 0.15% solution? 3. Solve the following concentration problems and show your work. • How many grams of NaCl are required to prepare 400 mL of a 1.50 M solution? There are 540 g of CH,,0, dissolved in 4 liters of solution. What is the molarity of this solution?
Chapter4: The Metric System
Section: Chapter Questions
Problem 1PP
Related questions
Question
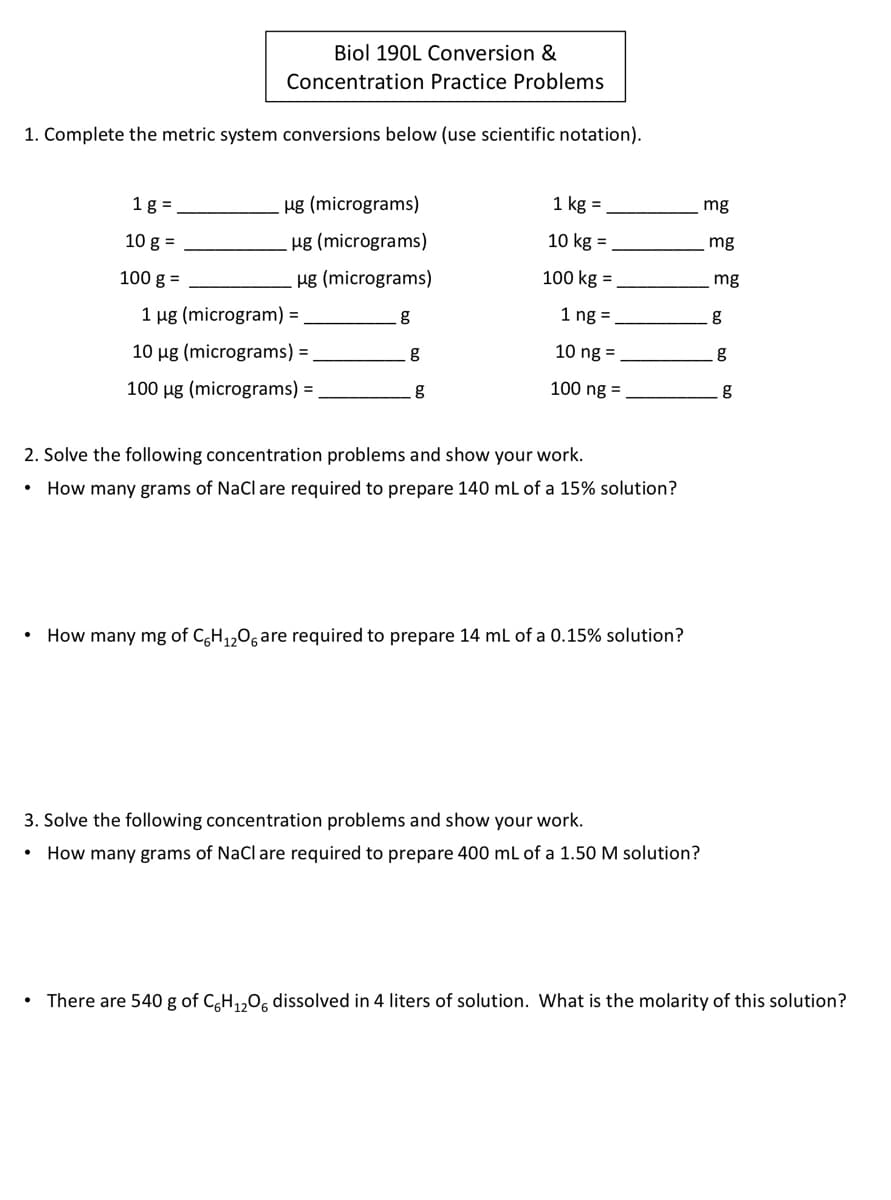
Transcribed Image Text:Biol 190L Conversion &
Concentration Practice Problems
1. Complete the metric system conversions below (use scientific notation).
1 g =
ug (micrograms)
1 kg =
mg
10 g =
Hg (micrograms)
10 kg =
mg
100 g =
Hg (micrograms)
100 kg =
mg
1 µg (microgram) =
1 ng =
g
10 ug (micrograms) =
g
10 ng =
100 ug (micrograms) =
g
100 ng =
2. Solve the following concentration problems and show your work.
How many grams of NaCl are required to prepare 140 ml of a 15% solution?
How many mg of C,H,,0, are required to prepare 14 ml of a 0.15% solution?
3. Solve the following concentration problems and show your work.
How many grams of NaCl are required to prepare 400 ml of a 1.50 M solution?
There are 540 g of CH,,0, dissolved in 4 liters of solution. What is the molarity of this solution?

Transcribed Image Text:Biol 190L Practice Problems
4. Solve the following concentration problems and show your work.
Calculate and describe how you would make 60 mL of a 0.8 M NaCl (working) solution from a
6 M stock solution.
Calculate how much (volume) of 0.8 M working solution could be made from 12 ml of 6 M
C6H1206 (stock) solution.
5. Solve the following concentration problem and show your work.
Calculate and circle which represents a higher concentration of NaCl : 1) 5 M or 2) 20%?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you



Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage



Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage
