By heat exchange, the butane fed into the reactor is brought to the reaction tempera- ture of 1000K It is desired to maintain an isothermal reactor How much heat must be added or removed per g mole of butadiene formed? Assume the pressurė is 100 kPa. What are some reasons why the heat load may be different from the value you calculate?
By heat exchange, the butane fed into the reactor is brought to the reaction tempera- ture of 1000K It is desired to maintain an isothermal reactor How much heat must be added or removed per g mole of butadiene formed? Assume the pressurė is 100 kPa. What are some reasons why the heat load may be different from the value you calculate?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
بروجكت
Transcribed Image Text:Draw
Design
Layout
References
Mailings
Review
View
Help
Table Design
Layout
O Commer
10
- A A Aa A
PFind
AaBbCcDc AaBbCcDc AaBbC
Replace
x, x
A - A -
,|1ニ|。
1 Normal
1 No Spac. Heading 1
Dictate
Sensitivity
Editor
R.
A Select -
Font
Paragraph
Styles
Editing
Voice
Sensitivity
Editor
Reu
5
. 8 .. I
Mini Frojeci sec 20
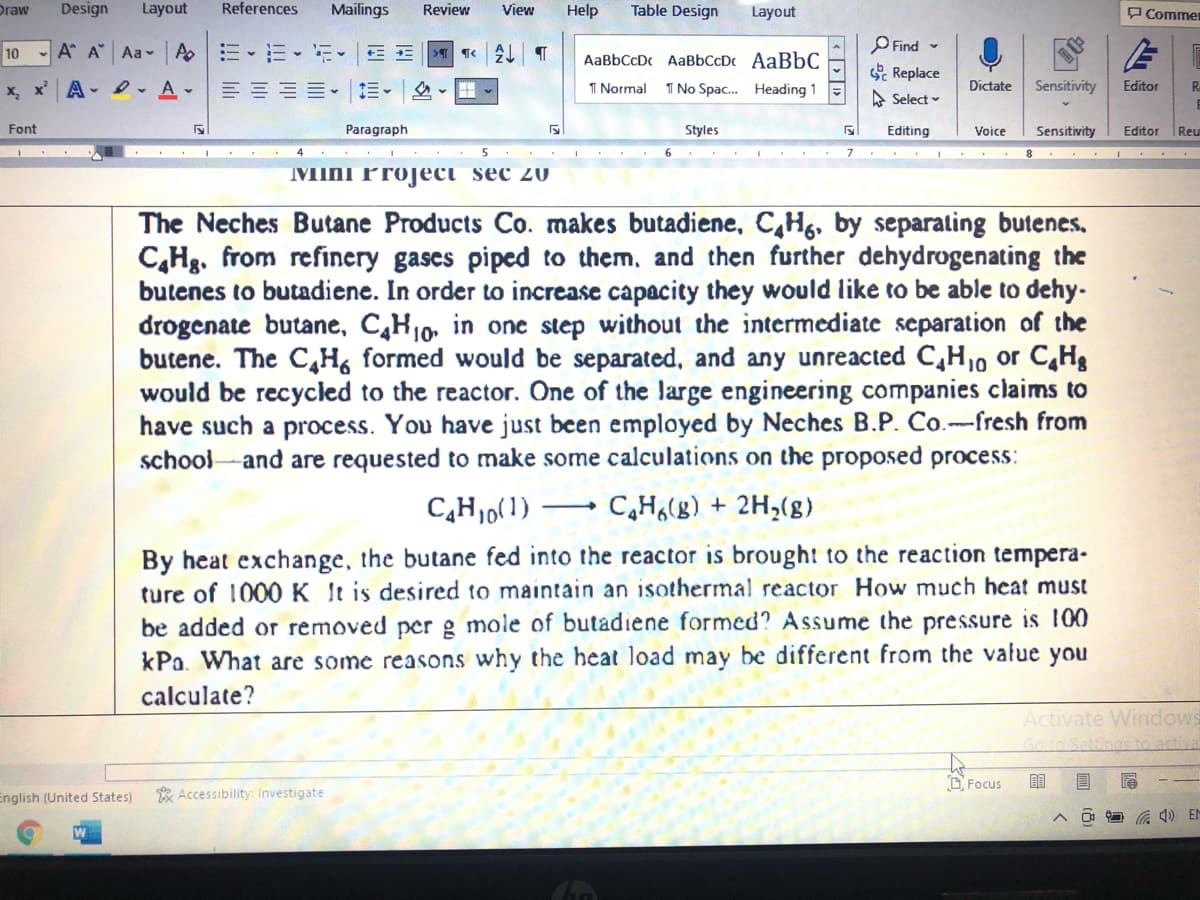
The Neches Butane Products Co. makes butadiene, C,Hg, by separating butenes.
C,Hg. from refinery gases piped to them, and then further dehydrogenating the
butenes to butadiene. In order to increase capacity they would like to be able to dehy-
drogenate butane, C,H10, in one step without the intermediate separation of the
butene. The C,H, formed would be separated, and any unreacted C,H,, or C,Hg
would be recycled to the reactor. One of the large engineering companies claims to
have such a process. You have just been employed by Neches B.P. Co.-fresh from
school-and are requested to make some calculations on the proposed process:
C,H,0(1) → C,H,(g) + 2H,(g)
By heat exchange, the butane fed into the reactor is brought to the reaction tempera-
ture of 1000K It is desired to maintain an isothermal reactor How much heat must
be added or removed per g mole of butadiene formed? Assume the pressure is l00
kPa. What are some reasons why the hecat load may be different from the value you
calculate?
Activate Windows
Go to Settinas to activa
D Focus
目
English (United States)
* Accessibility: Investigate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The