Chapter13: Aquatic Structures And Equipment
Section: Chapter Questions
Problem 2KA
Related questions
Question
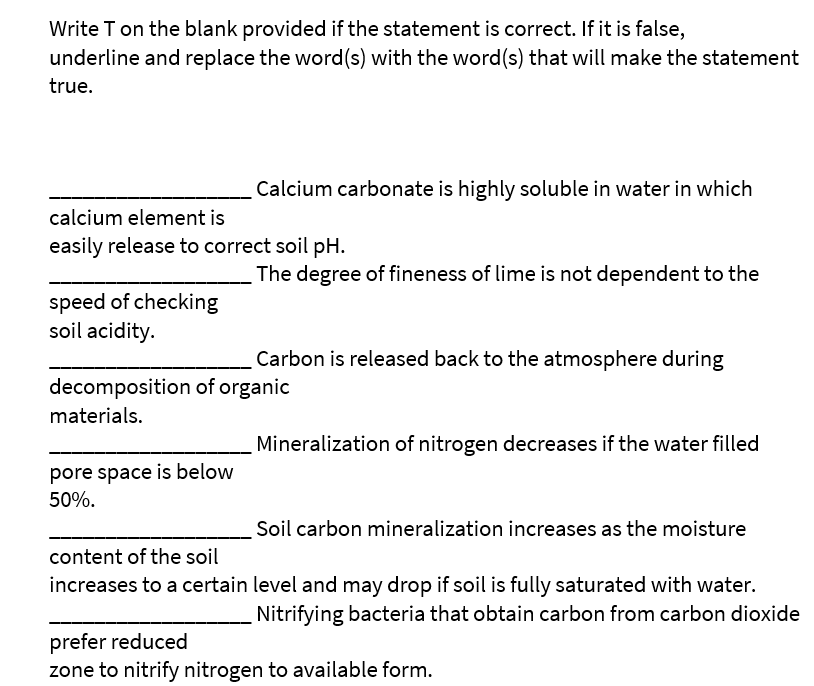
Transcribed Image Text:Write T on the blank provided if the statement is correct. If it is false,
underline and replace the word(s) with the word(s) that will make the statement
true.
Calcium carbonate is highly soluble in water in which
calcium element is
easily release to correct soil pH.
The degree of fineness of lime is not dependent to the
speed of checking
soil acidity.
Carbon is released back to the atmosphere during
decomposition of organic
materials.
Mineralization of nitrogen decreases if the water filled
pore space is below
50%.
Soil carbon mineralization increases as the moisture
content of the soil
increases to a certain level and may drop if soil is fully saturated with water.
Nitrifying bacteria that obtain carbon from carbon dioxide
prefer reduced
zone to nitrify nitrogen to available form.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you


Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax


Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax