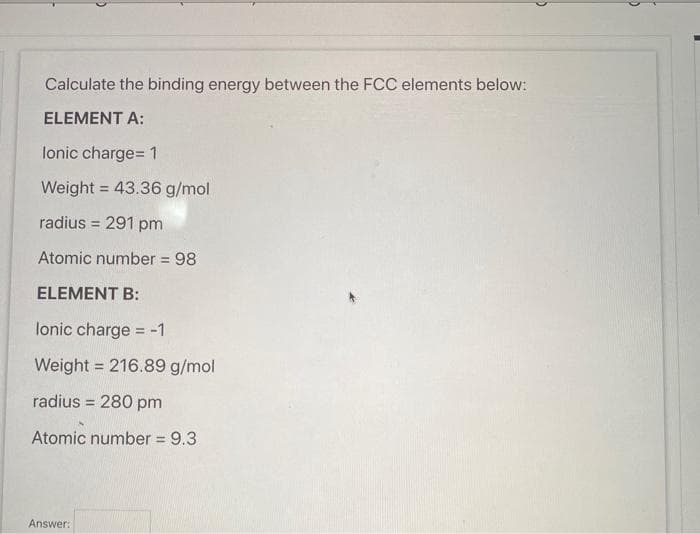
Calculate the binding energy between the FCC elements below: ELEMENT A: lonic charge= 1 Weight = 43.36 g/mol %3! radius = 291 pm Atomic number = 98 ELEMENT B: lonic charge = -1 Weight = 216.89 g/mol radius = 280 pm %3D Atomic number = 9.3
Q: Where are the nodes in the wavefunction for a particle confined to a box with 0 < x < a and n=3? (Se...
A: We need to determine:
Q: A Rydberg hydrogen atom is in the n=45 energy state. (a) What is the energy difference (in eV) betwe...
A: Part-a The initial energy state n1 = 45. The final energy state n2 = 46.
Q: *5-36. The beam of negligible weight is supported horizontally by two springs. If the beam is horizo...
A: Given: The diagram is as follows: Introduction: A force is a push or pulls upon an object resulting...
Q: If you add boiling water to a cup at room temperature, what would you expect the final equilibrium t...
A: According to the zeroth law of thermodynamics, when two objects with different temperatures are in t...
Q: A. A metal sphere has a coefficient of thermal expansion of is 9 x 10-6 °C-1. The internal diameter ...
A: Given, Coefficient of thermal expansion α=9×10-6 C-10diameter din=10.2 cm at T1=200Cdf=5.2 cm at Tf
Q: Q. A bead of mass m, which is attached to a vertical metal ring of radius R, reaches point D by pass...
A: When the bead of mass m is attached to a vertical ring of radius R and is dropped from point A, then...
Q: A horizontal force F pulls a 20-kg carton across the floor at constant speed. If the coefficient of ...
A: Given: The mass of the cartoon is m = 20 kg The distance the cartoon should move is d = 3 m The coef...
Q: When a capacitor is being charged, the instantaneous current i at time t is given by: i (10-e «C ), ...
A: Given, i=10-e-tRC
Q: I hope you can help me to solve this thank you
A: 1. average velocity=total distancetoatal time=20025=8m/s
Q: This problem involves a cylinder falling inside a pipe that is Giled with oil, as depicted in the fi...
A: Part a: For a steady flow, the net force on the cylinder must be zero. The area A of the moving surf...
Q: Thermodynamics Question! Please and thank you for all your help! Please make sure the answer is vali...
A: Work Done=(Force in the direction of displacement)×(Displacement)Only the component of force which i...
Q: Q/For the hook support shown, determine by trigonometry the magni- tude and direction of the resulta...
A: There are two forces acting on the hook, 300N and 200N 300 N making an angle 45° with the vertical v...
Q: As shown in the figure below, a 15-g bullet is fired horizontally into a 3-kg block of wood suspende...
A: Given: The mass of the bullet mb=15 g=0.015 kg. The mass of the wooden block mw=3 kg. The height of ...
Q: 1.12 When a bicycle rider is traveling at a speed of V = 24 mph, the power P she needs to supply is ...
A: Given data V=24 mph=10.73 m/secF=5 lbf=22.24 N
Q: cy.prove mather
A: Given: A cycle is drawn as,
Q: Find the half life of the radioactive element.
A: The half-life of a radioactive element is defined as the time at which the initial number of nuclei ...
Q: A large capacitance of 1.84 mF is needed for a certain application. (a) Calculate the area the paral...
A:
Q: What is the main difference in velocity or v-vs-t graphs between moving at constant speed and moving...
A: Introduction: Acceleration is the rate of change of the velocity of an object with respect to time.T...
Q: draw an idealized graph for both the v-vs-t graph and the corresponding a-vs-t graph for a cart that...
A: Given, initially, the cart slows down slowly, then stops, and then speeds up slowly. Let initially t...
Q: 7. A daredevil on a motorbike wants to jump over a river that is 25 m across using a rampat the edge...
A: according to problem range that has to be covered is R=25 meter let V is minimum velocity to reach t...
Q: Verify that for a harmonic oscillator, average potential energy <V> < E - for the quantum n...
A: The average energy of harmonic oscillation is : E=v+12hω Given, h=1m=1k=1 We know, ω=km=1 Thus, E=v+...
Q: A body projected upward from the level ground at an angle of 50 degrees with the horizontal has an i...
A:
Q: Uranium, an important component of both nuclear weapons and nuclear reactors, has two major isotopes...
A: Given that: Half life of U-238, T1=4.5×109 yearsHalf life of U-235, T2=0.7×109 yearsLet initially th...
Q: The nucleus of a certain atom has a mass of 8 x 10-25 kg and is at rest. The nucleus is radioactive ...
A: Given: Atom mass Ma=8×10-25kgMass of particle M=6.6×10-27kgspeed v=1.5×107kg To find: The recoil spe...
Q: The figure below shows a slab made of three different materials X, Y and Z having refractive indices...
A: A slab made of three different materials X, Y and Z having refractive indices nx, ny, and nz. The s...
Q: Galileo is often credited with the early discovery of four of Jupiter's many moons.The moons orbitin...
A: According to Kepler's law of harmonics (or Kepler's third law), the period of motion of a moon aroun...
Q: QUESTION 16.5, please
A: distance between two adjacent protons = 2fm=2×10-15m charge on proton q=1.6×10-19C mass of proton m=...
Q: The velocity of an object as a function of time is given by v=3t2+5t-12 m/s, where t is in seconds....
A:
Q: A 60 kg skier starts from rest at a height H = 33 m above the end of a ski jump ramp. If the skier ...
A: Solution given below:
Q: Their are two thin circular rings of radius R and charge -Q with their axes coinciding. One of the r...
A: The magnitude of electric field due to a point charge q can expressed by the following relation. E=1...
Q: Review The current-versus-voltage plot for a solar panel is shown in (Figure 1). Part A The short-ci...
A: Solution: We know that, In general, The short circuit current can be produced when its output volt...
Q: A delivery boy wishes to launch a 2.0-kg package up an inclined plane with sufficient speed to reach...
A: Solution Given Below:
Q: Consider an Ar atom trapped in a square box with a length of 1 m. Assume that the energy of the Ar a...
A: The energy of the particle in a box is given by Where m = mass of the particle, a = box length, ...
Q: Eg Q.1/ Prove that: ni = /NcNy e KT %3D Using Eg = Ec-Ev %3D
A: Semiconductors Semiconductors are those materials that are between conductors and insulators. There ...
Q: Complete the decay process that is showing below and naming the unknown product also find the kineti...
A: Concept used: During beta decay , three products are produced. Anti-neutrino is released during this...
Q: An airplane engine and the pylon that attaches it to the wing are idealized as shown below. Drive th...
A:
Q: This is a practice hw question not graded!!
A: In given problem: initial velcoity of fish =( 4 i+ 1j) m/sec final velcoity of fish =( 19 i - 7j) m/...
Q: In real recording situations there are multiple devices that each have their own sampling rate. An a...
A: Set up 1 Given: The sampling rate of the microphone is 48000 Hz. The sampling rate of audio processo...
Q: Where must an 800-N weight be hung on a uniform 100-N pole so that a boy at one end supports one- th...
A: The FBD of the system is as follows;
Q: In an ordinary television set, the electron beam consists of electrons shot horizontally at the tele...
A: Given Data : Speed : 5*107 m/s distance = 40 cm = 0.4 mein
Q: The figure shows capacitor 1 (C1 = 9.55 µF), capacitor 2 (C2 = 6.12 µF), and capacitor 3 (C3 = 7.93 ...
A:
Q: Consider the equation of total energy for a body falling freely is: E =mv?. V(x). Derive an equation...
A: E=12mv2+V(x) total energy of a body is constant in conservative field. so E is constant we know v=dx...
Q: A 2.0-kg block rests over a small hole on a table. A 15.0-g bullet is shot from below through the ho...
A: mass of block M=2kg mass of bullet m=0.015kg rise of (block+bullet) h= 1.3m let say velocity of bull...
Q: solar radiation power 3.95 * 10 ^ 33 erg.cm ^ (- 2) T ^ (- 4) effective temperature 5700 K, apparent...
A: The solar radiation power is, P=3.95×1033 erg/s =3.95×1033×10-7 J/sP=3.95×1026 J/s This power radia...
Q: 3. Consider the electric potential V generated by two pairs of positive and negative point charges o...
A:
Q: rq1-
A: Concept used: Refraction is the process in which light travels from one medium to another. Refracti...
Q: please answer section 2
A: The correct option is A.
Q: In same question I wanna know I remaining in place of I cavity??
A: Given, mass of disc = M radius of disc = R Let the mass density of disc is σ, Moment of inertia of...
Q: Please help answer questions 3, and 4 info: Imagine the scene based on your observations of American...
A: (3) Given: The final position is xf. The initial velocity is xi. The launch angle is θ. Introduction...
Q: A general aviation aircraft is flying at a geopotential altitude of 10,000 ft with a true airspeed o...
A: Given: h=10000 ft=3048 mtxs; s=0.05 lbft3
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
