Calculate the volume of helium needed in a hot air balloon to lift at least 100 kg of load. Density of He = 0.179 kg/m3, density of air = 1.29 kg/m3 (Answer: 90 m3) To solve the problem, which of the following equation/s can be used? Check as many that best applies to the solution.
Calculate the volume of helium needed in a hot air balloon to lift at least 100 kg of load. Density of He = 0.179 kg/m3, density of air = 1.29 kg/m3 (Answer: 90 m3) To solve the problem, which of the following equation/s can be used? Check as many that best applies to the solution.
Related questions
Question
Calculate the volume of helium needed in a hot air balloon to lift at least 100 kg of load.
Density of He = 0.179 kg/m3, density of air = 1.29 kg/m3
(Answer: 90 m3)
To solve the problem, which of the following equation/s can be used? Check as many that best applies to the solution.
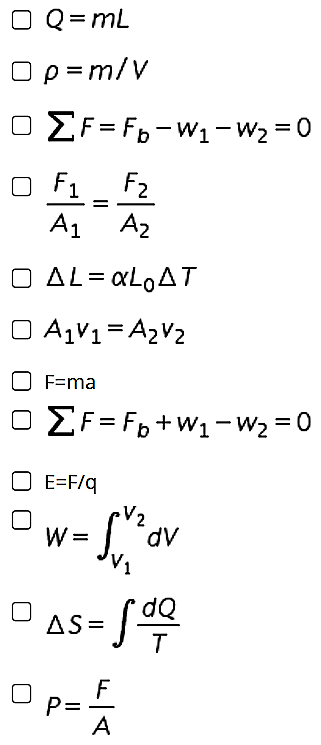
Transcribed Image Text:Q= mL
Ορ=m/V
O EF=F,-W1-W2 = 0
O F1 _ F2
A2
A1
O AL= «L0AT
O AV1=A2V2
F=ma
O EF = F6+W1-w2 = 0
O E=F/q
V2
W =
%3D
As = [d
Op-
T
F
P=
A

Transcribed Image Text:Fp=P fluidgV submerged
O Q= mcAT
O P= pgh
dU = dQ - dW or AU= Q- W
P2+pgh2+pvź =P1+pgh1+
e = 1-
Tn
1 |9192|
F =
4 TEO
ÞEDA = Ienc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
