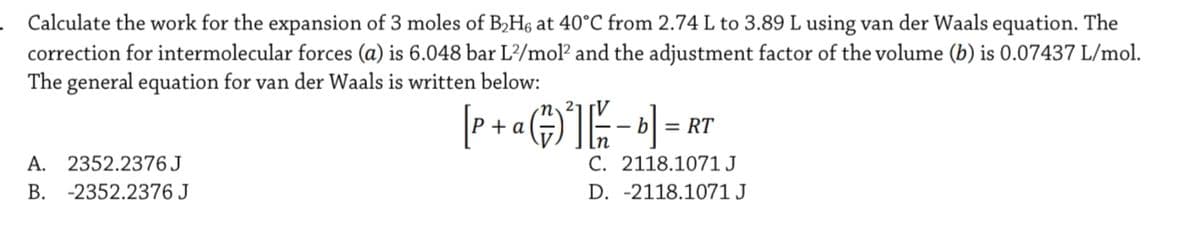
Calculate the work for the expansion of 3 moles of B,H6 at 40°C from 2.74 L to 3.89 L using van der Waals equation. The correction for intermolecular forces (a) is 6.048 bar L/mol? and the adjustment factor of the volume (b) is 0.07437 L/mol. The general equation for van der Waals is written below: + a -- b| = RT
Q: Problem An object of mnass m = 0.2 kg is hung from a spring whose spring constant is 80 Nm-1. The bo...
A: According to Newton's second law Fnet=ma...........1Here there are two forces acting on the spring-...
Q: A refrigerator has a coefficient of performance of 2. The ice (cice = 2090 J/kg.°C) tray compartment...
A: Given data, Performance coefficient of the refrigerator = 2 Cice = 2090 J/kg.oC Cwater = 4186 J/kg.o...
Q: An object is placed 47.5 cm from a concave spherical mirror with focal length of magnitude 22.0 cm. ...
A: Given: Object distance u= 47.5 cm Focal length of spherical mirror =22 cm To find: a) Ima...
Q: What are type I and type II superconductors?
A: Type I superconductors are also known as soft super conductors. The specimen is a normal conductor a...
Q: A bat is chasing a moth and it emits a sequence of clicks, 0.01 seconds apart. The bat hears the fir...
A: Given: The first echo is heard at 0.06 s. The second echo is heard at 0.075 s. The third echo is hea...
Q: A C1 = 12 μF capacitor is charged at 600 Volts. If we want to store the same electric charge but cha...
A: Given:C1=12 μFV1=600 VoltsV2=60 VoltsC2=?
Q: Two sinusoidal waves of wavelength A = 2/3 m and amplitude A = 6 cm and differing with their phase c...
A: GIven data: λ=23mA=6cmv=50m/s The frequency of the wave is given by: f=vλ=5023=75Hz Finding the ang...
Q: An optometrist has a patient who needs bifocals. The patient can clearly see an object when it is be...
A:
Q: A dog sits at the center of a disk that is rotating at a constant rate. The dog slowly moves to the ...
A: A dog sits at the center of a disk that is rotating at a constant rate. we know thatangular momentum...
Q: Two identical waves travel in the same direction, each with a wavelength A =1m %3D and speed v = 20 ...
A: Given: λ=1 mv=20 m/s
Q: A standing wave has the following wave-function: y(x,t) = O.2 sin(3tx) cos(12Ttt), where x and y are...
A: A standing wave is formed in a string fixed at both ends when two similar but oppositely directed wa...
Q: A disk of mass M and radius R rolls without slipping down a fixed inclined plane that makes an angle...
A: Subpart (a): Note: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit...
Q: Answer clearly
A: We know that the potential energy is given by V=mgh and the kinetic energy is given by K=12mv2 wher...
Q: onde
A: Given:- f = 50 Hz λ =1.2 m x = 0.5 m
Q: Hi please help: A block of wood with density of 0.421 g/cm^3 floats in some liquid of unknown densi...
A: Given, Wood density, ρ=0.421g/cm3 Let the V is the volume of the immersed block, ρ=mV0.421=mVV=m0.42...
Q: The long straight wire AB in the figure carries a current of 14.0A. The rectangular loop whose long ...
A: Current I1=14AI2=5Alength L=20cm=0.2mdistance a=2.6cm=0.026mand b=10cm=0.1m magnetic field B due to ...
Q: Suppose that a movie theater snack bar turns over its inventory of candy 3.9 times per month. (Round...
A: according to the given data, the movie theater turnover for the 370 boxes would be: 370*3.9 per mont...
Q: A cart with mass m2 = 13.5 kg is rolling to the left along a horizontal plane with a constant speed ...
A: Here, we apply conservation of momentum in the horizontal direction to get the final velocity.
Q: Determine the constants, A and B:2x-3/(x-3)(x-4) = A/x-3 + B/x-4
A: We solve the equations by comparision of terms.
Q: Two sinusoidal waves travelling in the same direction with the same amplitude, wavelength, and speed...
A: Given: Two sinusoidal waves traveling in the same direction with the same amplitude, wavelength, and...
Q: A sinusoidal function has a minimum point at (2,4) it has an amplitude of 3. Use this to determine t...
A: The amplitude of the sinusoidal function is 3. The minimum point of the function is at (2, 4). Thus,...
Q: Though what voltage must a proton be accelerated from rest in order for the percent error between th...
A: The classical kinetic energy is given by, KEC=12mv2 And the relativistic kinetic energy is given by,...
Q: Calculate the magnitude and direction of coulomb force on the last charge.
A: Given: Charge q1=6.00μCq2=1.50μCq3=-2.00μCDistance between charge q1 and q2 is r1=3.00 cmDistance be...
Q: In the diagram an electron is in motion but the direction is to be determined. The force on the elec...
A: The magnetic field in the figure is directed from the north pole of the magnet to the south pole, so...
Q: The distance between the first and the third nodes of a standing wave is 0.1 m, its maximum displace...
A: Given: The distance between first and third node is d=0.1 m. The maximum displacement of particle i...
Q: Problem 2 Consider the elastic pendulum shown in the figure below. The pendulum con- sists of a bob ...
A: Given:- (1) An elastic pendulum is also called swinging pendulum. When a mas...
Q: A uniform circular disk whose radius R is 17.8 cm is suspended as a physical pendulum from a point o...
A: (b) Given: The radius of the circular disk is 17.8 cm. Introduction: A time period is a time taken f...
Q: Pressurized water is often available at elevated temperatures and may be used for space heating or i...
A:
Q: give the given, required, formula, and complete solution of the problem
A: Given: The depth of core is 5 cm The flux is 0.005 WB The core permeability is 1000
Q: How long would it take to a person to melt a 125 cm3cube of ice at 0 oC if he holds the cube in his ...
A: Given data: The volume of the ice cube is V=125 cm3 The heat emitted by the person is P=200 W
Q: If water bottles are geometrically similar, how much more water will a bottle that is 50 cmtall hold...
A: According to question geometry of bottle are similar. So its radius also similar or can say it is sa...
Q: H.W.: Find actual Vo in the circuit in Figure below by using linearity property and the assumption t...
A: Given, Applied potential is, V = 20V Resistors are, R1 = 4kΩR2 = 4kΩR3 = 8kΩR4 = 12kΩR5 = 3kΩR6 = 6k...
Q: A standing wave on a stretched string with a tension force F_T and of length L= 2 %3D m has the foll...
A: The equation of the wave is given as, yx,t=0.1sin2πxcos100πt The standard wave equation is given as,...
Q: Four point charges are located at the corners of square 4 cm has been shown in figure Below, each ch...
A: Given: Side, a=4 cm= 4×10-2mAll charges have same magnitude, q=4nC=4×10-9C Ac=AB2+BC2AC=0.042+0.04...
Q: If sea level is 101350pa ,calculate the standard pressure at an altitude of 5000m using (a) the exac...
A: Given: Pa=101.35 Pa B=0.0065 k/m R=287 J/kg k Z=5000 m T0=15 degree C Formula used: P=Pa1-BZT0gRB P...
Q: kindly explain elaborately with reason not just putting formula thank you !
A: Ampere's law: According to this law the circulation ∮B→·dl→ of a resultant magnetic field along a cl...
Q: A standing wave on a stretched string fixed at both ends is described by: y(x,t) = 0.1 sin(2Ttx) cos...
A: Given: Equation is described as y(x,t)=0.1sin(2πx)cos(100πt) The string's length is 1 m The distance...
Q: Two identical sinusoidal waves of amplitude A and wavelengths of 2.00 m travel in the +x direction a...
A: Concept used: Destructive interference of the waves occur when they are out of phase. Constructive i...
Q: (c) Write down the reaction describing the decay of Na to ỗNe. Calcula
A: Given: Atomic mass of N1122a is mNa=21.99444u Atomic mass of N1022e is mNe=21.99139u We have to writ...
Q: A ladder of length L and negligible weight leans against a frictionless vertical wall making an angl...
A: Given : Length of the ladder=L angle=θ Coefficient of friction= μs Weight of the man =W
Q: A standing wave on a stretched string with a tension force F Tand of length L = 2 m has the followin...
A: The standing wave on the stretched spring is defined by the equation as, yx,t=0.1sin2πxcos100πt By c...
Q: Two transverse sinusoidal waves combining in a string are described by the wave functions y1 = 0.02 ...
A: Given, y1=0.02sin4πx+πty2=0.02cos4πx-πt Compare with y=Asinkx+ωt So, wave angular number, k=4π Angul...
Q: Consider a shape with Radius = 3.5cm Total charge 0.66µC Electric flux in the surface (the round sur...
A: Given: ϕ=9.8×104Nm2/C
Q: Two identical sinusoidal waves with wavelengths of 1.5 m travel in the same direction at a speed of ...
A: Given : λ=1.5m v=10m/s A'=3A The amplitude of the two waves is A. Let th...
Q: A ray of white light traveling through air enters a triangular prism that has an index of refraction...
A: Given: The index of refraction for red light is nr=1.512. The index of refraction for violet right ...
Q: A student wants to establish a standing wave on a wire 1.8 m long clamped at both ends. If the wave ...
A: A standing wave is formed when two waves superimpose in a confined medium. These two progressive wav...
Q: Please help me
A: (4) Given: The radius of the solid insulating sphere is 4.4 cm. The charge density on the inner sphe...
Q: Saslg älbäi The paragraph below is an example * of unsolicited job application letter I'm interested...
A: It is a document you send with your resume for a position that isn’t officially open. Just like a c...
Q: What's the answer?
A: The Diagram 2 above shows the Doppler effect. The sound waves from the police car siren travel outwa...
Q: Advanced Physics Question
A: Hello. Since you have posted multiple questions and not specified which question needs to be solved,...
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
