can you help me solve this, this is what i have so far but is there a different way of doing this. please type solution in word. calculate how much KNO3 can be removed as solid (filtered) from a 1-kilogram solution of 60 wt% KNO3 in water (40 wt%) at 65 °C, when it is cooled to 25 °C. Note that the 60 wt% value is given on a basis of total solution mass, which is different than the values presented in the graph.
can you help me solve this, this is what i have so far but is there a different way of doing this. please type solution in word. calculate how much KNO3 can be removed as solid (filtered) from a 1-kilogram solution of 60 wt% KNO3 in water (40 wt%) at 65 °C, when it is cooled to 25 °C. Note that the 60 wt% value is given on a basis of total solution mass, which is different than the values presented in the graph.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
can you help me solve this, this is what i have so far but is there a different way of doing this. please type solution in word.
calculate how much KNO3 can be removed as solid (filtered) from a 1-kilogram solution of 60 wt% KNO3 in water (40 wt%) at 65 °C, when it is cooled to 25 °C. Note that the 60 wt% value is given on a basis of total solution mass, which is different than the values presented in the graph.
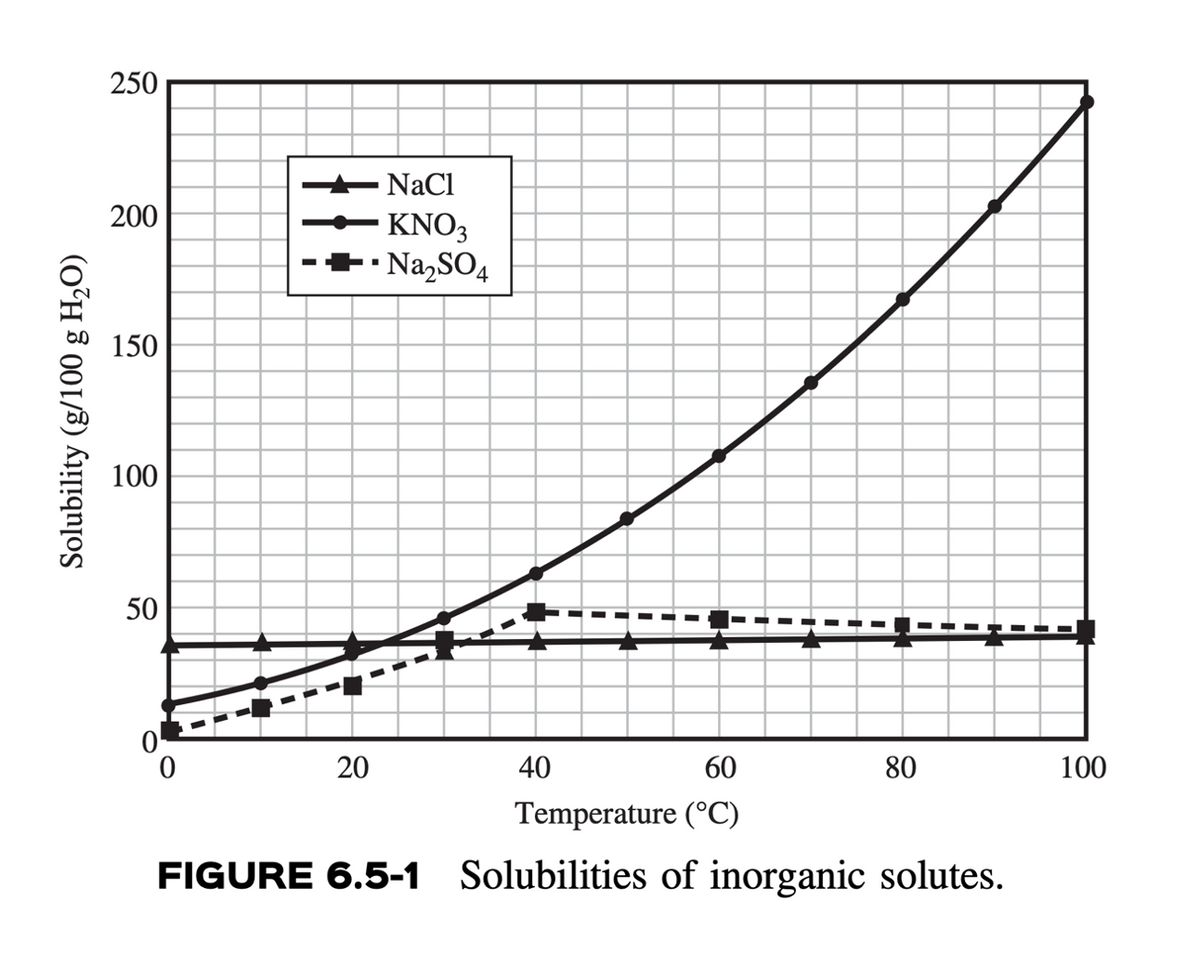
Transcribed Image Text:Solubility (g/100 g H₂O)
250
200
150
NaCl
KNO3
Na2SO4
100
50
20
40
60
60
80
100
Temperature (°C)
FIGURE 6.5-1 Solubilities of inorganic solutes.
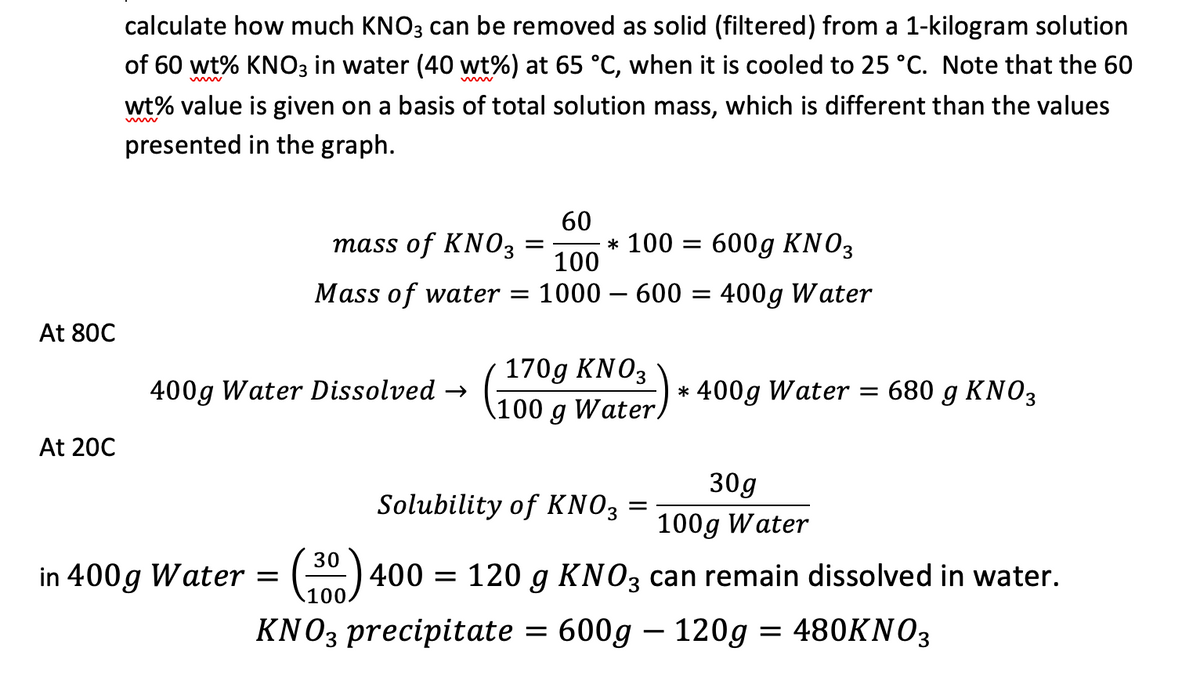
Transcribed Image Text:calculate how much KNO3 can be removed as solid (filtered) from a 1-kilogram solution
of 60 wt% KNO3 in water (40 wt%) at 65 °C, when it is cooled to 25 °C. Note that the 60
www
www
www
wt% value is given on a basis of total solution mass, which is different than the values
presented in the graph.
60
mass of KNO3
=
100
Mass of water =
* 100 = 600g KNO3
: 1000 - 600 =
400g Water
At 80C
400g Water Dissolved →
170g KNO3
100 g Water,
* 400g Water = 680 g KNO3
At 20C
Solubility of KNO3 =
30g
100g Water
in 400g Water = (30) 400 = 120 g KNO3 can remain dissolved in water.
100
KNO3 precipitate = 600g - 120g = 480KNO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The