Chemical Engineering: Material Balance
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Use the atomic species balance for this. Chemical Engineering: Material Balance.
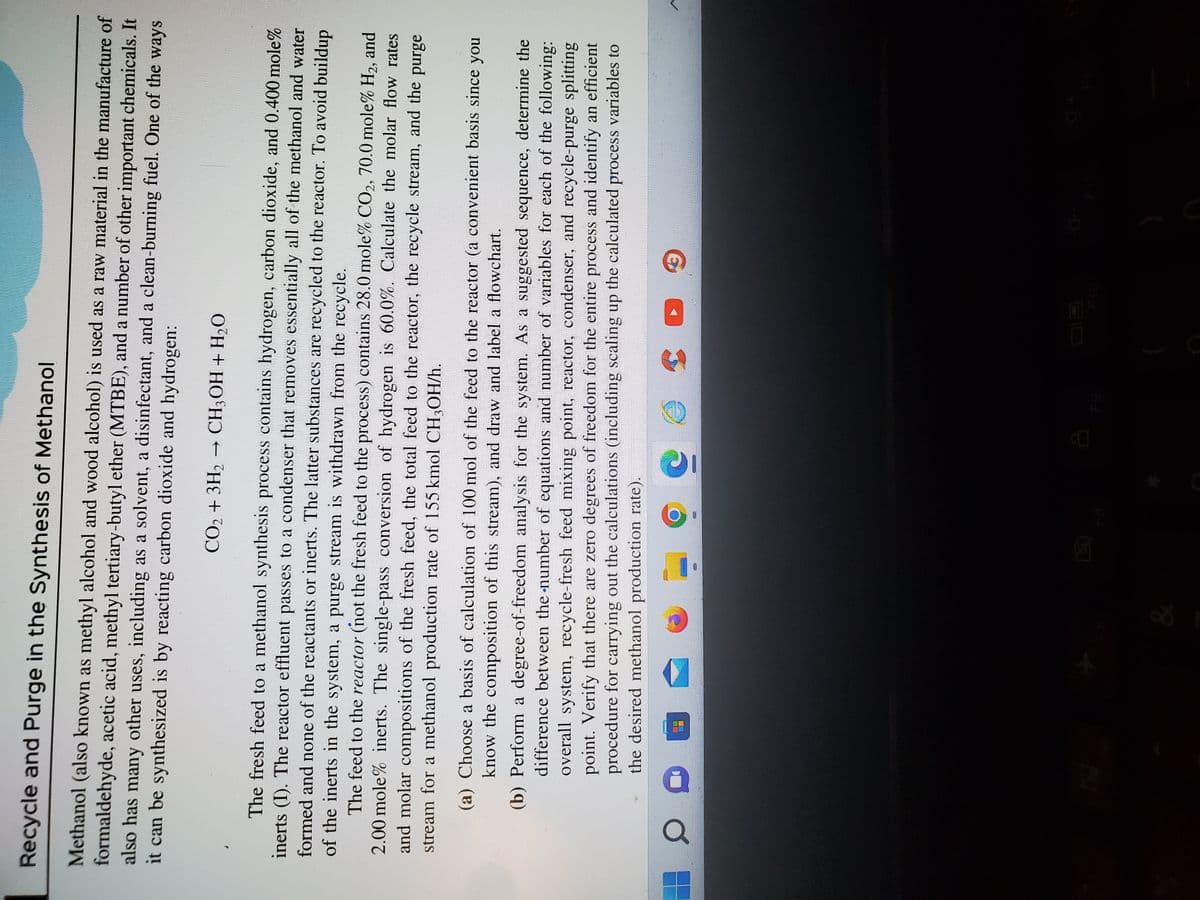
Transcribed Image Text:Recycle and Purge in the Synthesis of Methanol
Methanol (also known as methyl alcohol and wood alcohol) is used as a raw material in the manufacture of
formaldehyde, acetic acid, methyl tertiary-butyl ether (MTBE), and a number of other important chemicals. It
also has many other uses, including as a solvent, a disinfectant, and a clean-burning fuel. One of the ways
it can be synthesized is by reacting carbon dioxide and hydrogen:
CO₂ + 3H₂ CH3OH + H₂O
-
The fresh feed to a methanol synthesis process contains hydrogen, carbon dioxide, and 0.400 mole%
inerts (I). The reactor effluent passes to a condenser that removes essentially all of the methanol and water
formed and none of the reactants or inerts. The latter substances are recycled to the reactor. To avoid buildup
of the inerts in the system, a purge stream is withdrawn from the recycle.
The feed to the reactor (not the fresh feed to the process) contains 28.0 mole% CO2, 70.0 mole% H₂, and
2.00 mole% inerts. The single-pass conversion of hydrogen is 60.0%. Calculate the molar flow rates
and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream, and the purge
stream for a methanol production rate of 155 kmol CH3OH/h.
(a) Choose a basis of calculation of 100 mol of the feed to the reactor (a convenient basis since you
know the composition of this stream), and draw and label a flowchart.
(b) Perform a degree-of-freedom analysis for the system. As a suggested sequence, determine the
difference between the number of equations and number of variables for each of the following:
overall system, recycle-fresh feed mixing point, reactor, condenser, and recycle-purge splitting
point. Verify that there are zero degrees of freedom for the entire process and identify an efficient
procedure for carrying out the calculations (including scaling up the calculated process variables to
the desired methanol production rate).
€
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The