T(deg C) y P(bar) 62.5 0.0817 0.05 1 62.82 0.139 0.1 1 63.83 0.2338 0.15 1 64.3 0.3162 0.2 1 64.37 0.3535 0.3 1 64.35 0.3888 0.4 1 64.02 0.4582 0.5 1 63.33 0.5299 0.6 1 62.23 0.6106 0.7 1 60.72 0.7078 0.8 1 58.71 0.8302 0.9 1 57.48 0.9075 0.95 1 The Antoine equation parameters are given below (liquid-vapor equation): Log10P(mm Hg) = A- B/(T(deg C)+C) (valid over I and P range of this problem) %3D chloroform acetone А 6.95465 7.02447 В 1170.966 1161 C 226.232 224 a. Plot experimental data on a Txy diagram (P= 760 mm Hg). b. Which compound is more volatile when in a pure state? Plot predicted vapor and liquid equilibrium behavior based on Raoult's Law (also known as Lewis/Randall relationship) using the vapor pressures determined from the Antoine equation (plot bubble and dew lines). Write out equations used to solve this step when turning in HW. d. Compare the experimental data with the Raoult's Law estimation; is this a positive or negative deviation from Raoult's Law? Is there a boiling maximum or С. minimum? e. Are the interspecies forces attractive or repulsive compared to forces in pure system? f. What is the liquid and vapor phase composition at the azeotrope? g. You have a 100% liquid mixture with acetone comprising 20% of the total moles; the mixture is heated until it starts to boil; What is the T? What is the composition of the first bubble? 1. at what T does the last drop evaporate? What is the composition of the last drop? 2. what is the composition of the mixture when it is 100% vapor? h. assuming the Modified Raoult's Law is appropriate, plot the activity coefficients for both species (1 and 2) as a function of X1, are the activity coefficients greater than or less than 1 at X1 = 0.5? Does this suggest attractive or repulsive
T(deg C) y P(bar) 62.5 0.0817 0.05 1 62.82 0.139 0.1 1 63.83 0.2338 0.15 1 64.3 0.3162 0.2 1 64.37 0.3535 0.3 1 64.35 0.3888 0.4 1 64.02 0.4582 0.5 1 63.33 0.5299 0.6 1 62.23 0.6106 0.7 1 60.72 0.7078 0.8 1 58.71 0.8302 0.9 1 57.48 0.9075 0.95 1 The Antoine equation parameters are given below (liquid-vapor equation): Log10P(mm Hg) = A- B/(T(deg C)+C) (valid over I and P range of this problem) %3D chloroform acetone А 6.95465 7.02447 В 1170.966 1161 C 226.232 224 a. Plot experimental data on a Txy diagram (P= 760 mm Hg). b. Which compound is more volatile when in a pure state? Plot predicted vapor and liquid equilibrium behavior based on Raoult's Law (also known as Lewis/Randall relationship) using the vapor pressures determined from the Antoine equation (plot bubble and dew lines). Write out equations used to solve this step when turning in HW. d. Compare the experimental data with the Raoult's Law estimation; is this a positive or negative deviation from Raoult's Law? Is there a boiling maximum or С. minimum? e. Are the interspecies forces attractive or repulsive compared to forces in pure system? f. What is the liquid and vapor phase composition at the azeotrope? g. You have a 100% liquid mixture with acetone comprising 20% of the total moles; the mixture is heated until it starts to boil; What is the T? What is the composition of the first bubble? 1. at what T does the last drop evaporate? What is the composition of the last drop? 2. what is the composition of the mixture when it is 100% vapor? h. assuming the Modified Raoult's Law is appropriate, plot the activity coefficients for both species (1 and 2) as a function of X1, are the activity coefficients greater than or less than 1 at X1 = 0.5? Does this suggest attractive or repulsive
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
non ideal mixture of acetone(1) and chloroform(2)
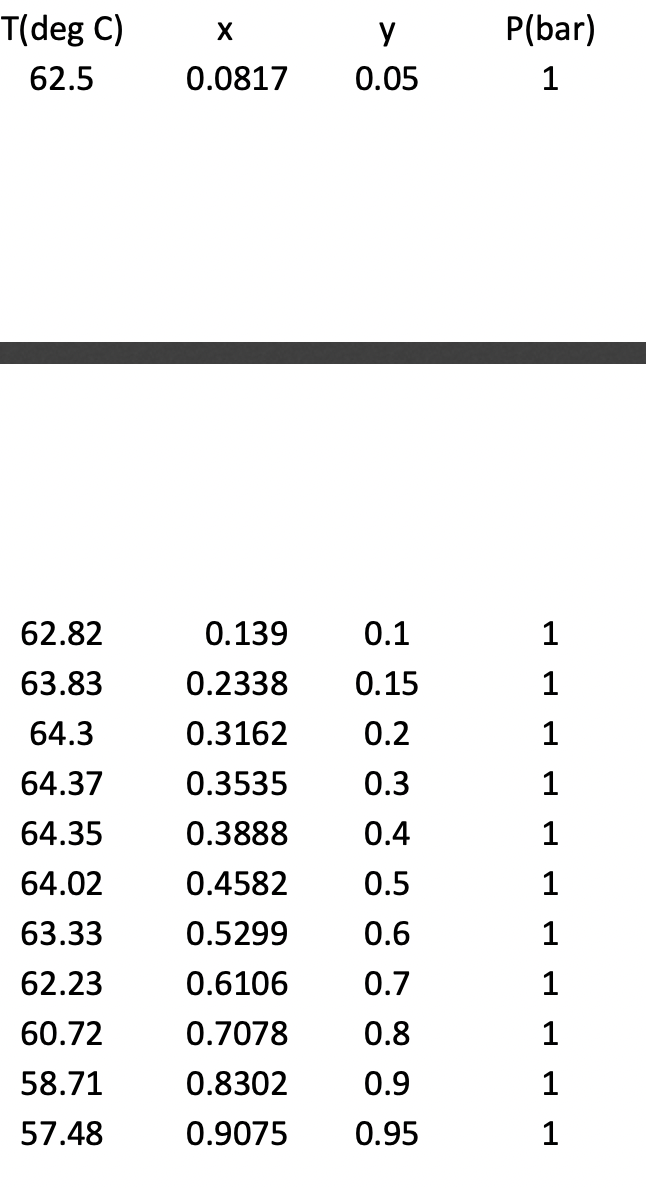
Transcribed Image Text:T(deg C)
y
P(bar)
62.5
0.0817
0.05
1
62.82
0.139
0.1
1
63.83
0.2338
0.15
1
64.3
0.3162
0.2
1
64.37
0.3535
0.3
1
64.35
0.3888
0.4
1
64.02
0.4582
0.5
1
63.33
0.5299
0.6
1
62.23
0.6106
0.7
1
60.72
0.7078
0.8
1
58.71
0.8302
0.9
1
57.48
0.9075
0.95
1

Transcribed Image Text:The Antoine equation parameters are given below (liquid-vapor equation):
Log10P(mm Hg) = A- B/(T(deg C)+C) (valid over I and P range of this problem)
%3D
chloroform
acetone
А
6.95465
7.02447
В
1170.966
1161
C
226.232
224
a. Plot experimental data on a Txy diagram (P= 760 mm Hg).
b. Which compound is more volatile when in a pure state?
Plot predicted vapor and liquid equilibrium behavior based on Raoult's Law (also
known as Lewis/Randall relationship) using the vapor pressures determined from
the Antoine equation (plot bubble and dew lines). Write out equations used to
solve this step when turning in HW.
d. Compare the experimental data with the Raoult's Law estimation; is this a
positive or negative deviation from Raoult's Law? Is there a boiling maximum or
С.
minimum?
e. Are the interspecies forces attractive or repulsive compared to forces in pure
system?
f. What is the liquid and vapor phase composition at the azeotrope?
g. You have a 100% liquid mixture with acetone comprising 20% of the total moles;
the mixture is heated until it starts to boil; What is the T? What is the
composition of the first bubble?
1. at what T does the last drop evaporate? What is the
composition of the last drop?
2. what is the composition of the mixture when it is 100% vapor?
h. assuming the Modified Raoult's Law is appropriate, plot the activity coefficients
for both species (1 and 2) as a function of X1, are the activity coefficients greater
than or less than 1 at X1 = 0.5? Does this suggest attractive or repulsive
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The