7-31. In the air dryer illustrated in Figure 7.31, part of the effluent air stream is to be recycled in an effort to control the inlet humidity. The solids entering the dryer (Stream #3) contain 20 % water on a mass basis and the mass flow rate of the wet solids entering the dryer is 1000 lb/h. The dried solids (stream #4) are to contain a maximum of 5 % water on a mass basis. The partial pressure of water vapor in the fresh air entering the system (Stream #1) is equivalent to 10 mm Hg and the partial pressure in the air leaving the dryer (Stream #5) must not exceed 200 mm Hg. In this particular problem the flow rate of the recycle stream (stream #6) is to be regulated so that the partial pressure of water vapor in the air entering the dryer is equivalent to 50 mm Hg. For this condition, calculate the total molar flow rate of fresh air entering the system (Stream #1) and the total molar flow rate of the recycle stream (Stream #6). Assume that the process operates at atmospheric pressure (760 mm Hg). recycle stream fresh air Dryer wet solids 20% water dry solids 5% water Figure 7.31. Air dryer with recycle stream
7-31. In the air dryer illustrated in Figure 7.31, part of the effluent air stream is to be recycled in an effort to control the inlet humidity. The solids entering the dryer (Stream #3) contain 20 % water on a mass basis and the mass flow rate of the wet solids entering the dryer is 1000 lb/h. The dried solids (stream 4) are to contain a maximum of 5 % water on a mass basis. The partial pressure of water vapor in the fresh air entering the system (Stream #1) is equivalent to 10 mm Hg and the partial pressure in the air leaving the dryer (Stream #5) must not exceed 200 mm Hg. In this particular problem the flow rate of the recycle stream (stream #6) is to be regulated so that the partial pressure of water vapor in the air entering the dryer is equivalent to 50 mm Hg. For this condition, calculate the total molar flow rate of fresh air entering the system (Stream #1) and the total molar flow rate of the recycle stream (Stream #6). Assume that the process operates at atmospheric pressure (760 mm Hg). e recycle stream ® fresh air Dryer dry solids wet solids 20% water 5% water Figure 7.31. Air dryer with recycle stream
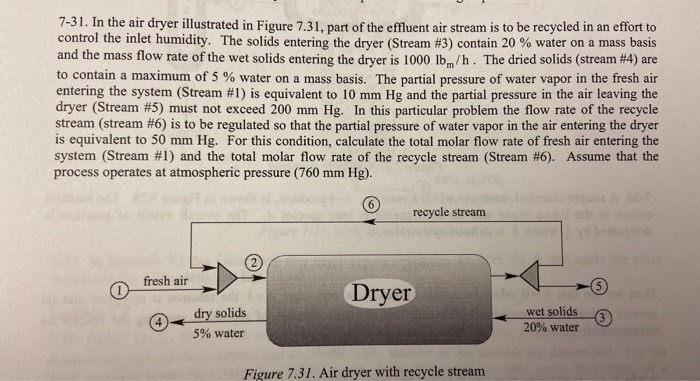
The mole fraction of water in stream one, stream two, and stream five are determined. The amount of solid in stream three and stream four are the same as water is only evaporating. The amount of water evaporated is determined. Water mass balance at the mixer is considered. In this system, the amount of dry air leaving is equal to the amount of dry air entering. The water component balance is considered to obtain fresh air flow rate and recycle flow rate.
Step by step
Solved in 2 steps









