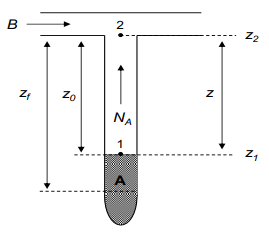
2 B Z2 Zo NA 1 z, A RTPBMPA(2;-20) 2 MĄDABP(PA1-PA2) (Hint: They used same equation for their calculations Zf+2o
Equations and Inequations
Equations and inequalities describe the relationship between two mathematical expressions.
Linear Functions
A linear function can just be a constant, or it can be the constant multiplied with the variable like x or y. If the variables are of the form, x2, x1/2 or y2 it is not linear. The exponent over the variables should always be 1.
Seda, Ecem and Nurdan tried to find diffusion coefficient of acetone in air all of them followed the procedure given in the GMÜ 382 lab. Manuel with small differences.
Seda conducted the experiment at 40oC. Ecem used a longer T tube (“z” distance is higher than other experiments). Nurdan conducted the experiment for 210 minutes and took data every 10 minutes.
After completing their experiments, they compared the results with each other. Do expect them to find same results support your answer with literature data and/or mathematical expressions do not forget to give reference for your answer.
(Hint: They used same equation for their calculations
Step by step
Solved in 4 steps with 2 images









