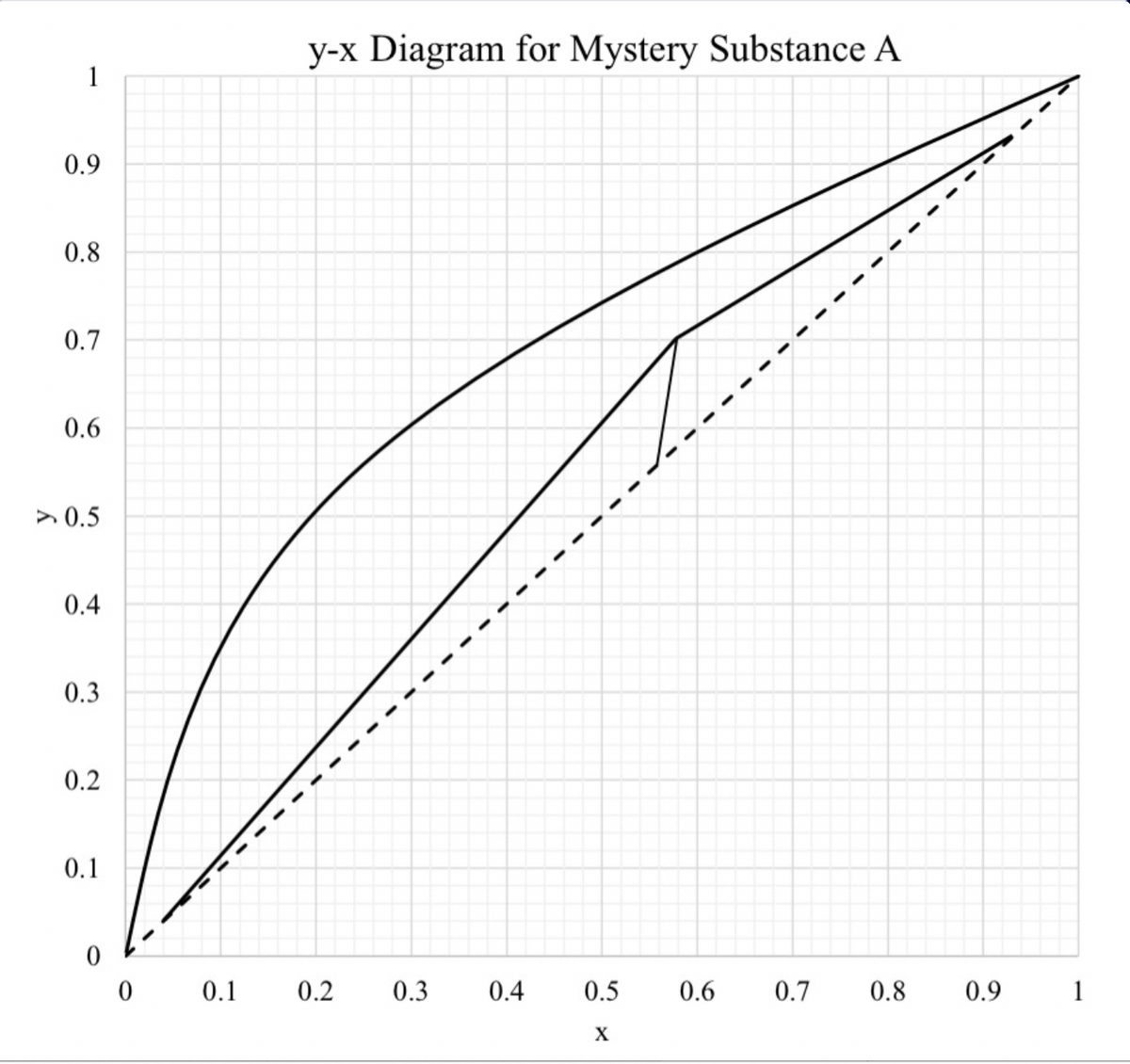
y-x Diagram for Mystery Substance A 1 0.9 0.8 0.7 0.6 > 0.5 0.4 0.3 0.2 0.1 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 X 1. You have just started working for Azeotrope Separations Co. and you have been placed in a project designing a distillation column to separate mystery substances A and B. Your predecessor left you the following incomplete McCabe-Thiele diagram (next page). Use this diagram to answer the following questions. You may assume that the column operates at constant pressure. a) What are xp, xw, and xp? What are the reflux and boil-up ratios? b) Your boss wants you to operate the column with a 100 kg mol/h feed. What are the flowrates of the bottoms and distillate? c) What is q? What does the slope of the q-line tell you about the feed quality? d) Complete the McCabe-Thiele diagram by stepping off stages. What are the theoretical number of stages? Where should the feed tray go? e) You find specifications left by your predecessor that say that the feed should be at stage 6. Assuming you have the same number of stages in part d, redraw the stages to account for this new feed location. Is the desired bottoms composition still achieved? f) You begin to suspect that your predecessor did not pay attention in CBE 371. You find a handwritten note that says “Increasing the reflux ratio (i) decreases the number of trays and (ii) increases distillate flow rate." Is this statement true? If so, which part(s)? Justify your answer. g) You find a draft of a McCabe-Thiele diagram where your predecessor was counting the number of stages when the reflux ratio was at Rmin. What will be the number of stages if the column is at Rmin? Explain your reasoning. (Are your suspicions about your predecessor confirmed?)
y-x Diagram for Mystery Substance A 1 0.9 0.8 0.7 0.6 > 0.5 0.4 0.3 0.2 0.1 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 X 1. You have just started working for Azeotrope Separations Co. and you have been placed in a project designing a distillation column to separate mystery substances A and B. Your predecessor left you the following incomplete McCabe-Thiele diagram (next page). Use this diagram to answer the following questions. You may assume that the column operates at constant pressure. a) What are xp, xw, and xp? What are the reflux and boil-up ratios? b) Your boss wants you to operate the column with a 100 kg mol/h feed. What are the flowrates of the bottoms and distillate? c) What is q? What does the slope of the q-line tell you about the feed quality? d) Complete the McCabe-Thiele diagram by stepping off stages. What are the theoretical number of stages? Where should the feed tray go? e) You find specifications left by your predecessor that say that the feed should be at stage 6. Assuming you have the same number of stages in part d, redraw the stages to account for this new feed location. Is the desired bottoms composition still achieved? f) You begin to suspect that your predecessor did not pay attention in CBE 371. You find a handwritten note that says “Increasing the reflux ratio (i) decreases the number of trays and (ii) increases distillate flow rate." Is this statement true? If so, which part(s)? Justify your answer. g) You find a draft of a McCabe-Thiele diagram where your predecessor was counting the number of stages when the reflux ratio was at Rmin. What will be the number of stages if the column is at Rmin? Explain your reasoning. (Are your suspicions about your predecessor confirmed?)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:y-x Diagram for Mystery Substance A
1
0.9
0.8
0.7
0.6
> 0.5
0.4
0.3
0.2
0.1
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
X

Transcribed Image Text:1. You have just started working for Azeotrope Separations Co. and you have been placed in a
project designing a distillation column to separate mystery substances A and B. Your predecessor
left you the following incomplete McCabe-Thiele diagram (next page). Use this diagram to
answer the following questions. You may assume that the column operates at constant pressure.
a) What are xp, xw, and xp? What are the reflux and boil-up ratios?
b) Your boss wants you to operate the column with a 100 kg mol/h feed. What are the flowrates
of the bottoms and distillate?
c) What is q? What does the slope of the q-line tell you about the feed quality?
d) Complete the McCabe-Thiele diagram by stepping off stages. What are the theoretical
number of stages? Where should the feed tray go?
e) You find specifications left by your predecessor that say that the feed should be at stage 6.
Assuming you have the same number of stages in part d, redraw the stages to account for this
new feed location. Is the desired bottoms composition still achieved?
f) You begin to suspect that your predecessor did not pay attention in CBE 371. You find a
handwritten note that says “Increasing the reflux ratio (i) decreases the number of trays and
(ii) increases distillate flow rate." Is this statement true? If so, which part(s)? Justify your
answer.
g) You find a draft of a McCabe-Thiele diagram where your predecessor was counting the
number of stages when the reflux ratio was at Rmin. What will be the number of stages if the
column is at Rmin? Explain your reasoning. (Are your suspicions about your predecessor
confirmed?)
Expert Solution
Step 1
Dear User, According to Bartleby policy ,we are authorized to solve only 3-subparts, kindly submit other parts separately to get it answered. thank you.
for McCabe-Thiele Method, we know that
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The