closed system consisting of 2 lb of a gas undergoes a process during which the relation between pressure and volume is pV" = nstant. The process begins with p₁= 15 lbf/in.², v₁ = 1.25 ft³/lb and ends with p2 = 46 lbf/in.², v₂ = 0.5 ft³/lb. Determine (a) the lume, in ft3, occupied by the gas at states 1 and 2 and (b) the value of n. Determine the volume, in ft3, occupied by the gas at states 1 and 2. V₁= V2 = Determine the value of n. ft³ ft³
closed system consisting of 2 lb of a gas undergoes a process during which the relation between pressure and volume is pV" = nstant. The process begins with p₁= 15 lbf/in.², v₁ = 1.25 ft³/lb and ends with p2 = 46 lbf/in.², v₂ = 0.5 ft³/lb. Determine (a) the lume, in ft3, occupied by the gas at states 1 and 2 and (b) the value of n. Determine the volume, in ft3, occupied by the gas at states 1 and 2. V₁= V2 = Determine the value of n. ft³ ft³
Related questions
Question
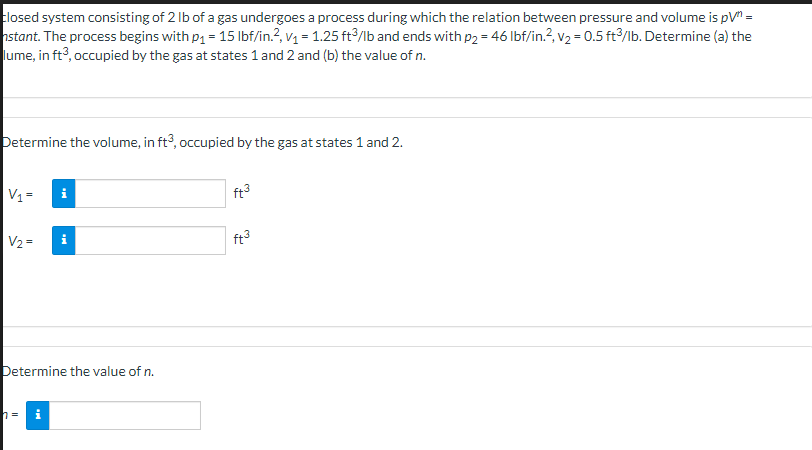
Transcribed Image Text:closed system consisting of 2 lb of a gas undergoes a process during which the relation between pressure and volume is pV" =
nstant. The process begins with p₁= 15 lbf/in.², v₁ = 1.25 ft³/lb and ends with p2 = 46 lbf/in.², v₂ = 0.5 ft³/lb. Determine (a) the
lume, in ft3, occupied by the gas at states 1 and 2 and (b) the value of n.
Determine the volume, in ft3, occupied by the gas at states 1 and 2.
V₁=
V2 =
Determine the value of n.
ft³
ft³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images
