Compound A diffuses through a 4 cm long tube and reacts as it diffuses. The equation governing diffusion with reaction along the distance x of the tube (in cm) is given by: d²A D dx2 - kA = 0 At one end of the tube, there is a large source of A at a concentration of 0.1 M. At the other end of the tube there is an adsorbent material that quickly absorbs any A, making the concentration of A zero. If D = 1.5 x 10-6 cm? s-1 and k=6 x 10-6 s-1, i. What is the general solution for the concentration of A as a function of distance in the tube? ii. What would be the concentration of A at lcm distance from the source?
Compound A diffuses through a 4 cm long tube and reacts as it diffuses. The equation governing diffusion with reaction along the distance x of the tube (in cm) is given by: d²A D dx2 - kA = 0 At one end of the tube, there is a large source of A at a concentration of 0.1 M. At the other end of the tube there is an adsorbent material that quickly absorbs any A, making the concentration of A zero. If D = 1.5 x 10-6 cm? s-1 and k=6 x 10-6 s-1, i. What is the general solution for the concentration of A as a function of distance in the tube? ii. What would be the concentration of A at lcm distance from the source?
Related questions
Question
Asap tq
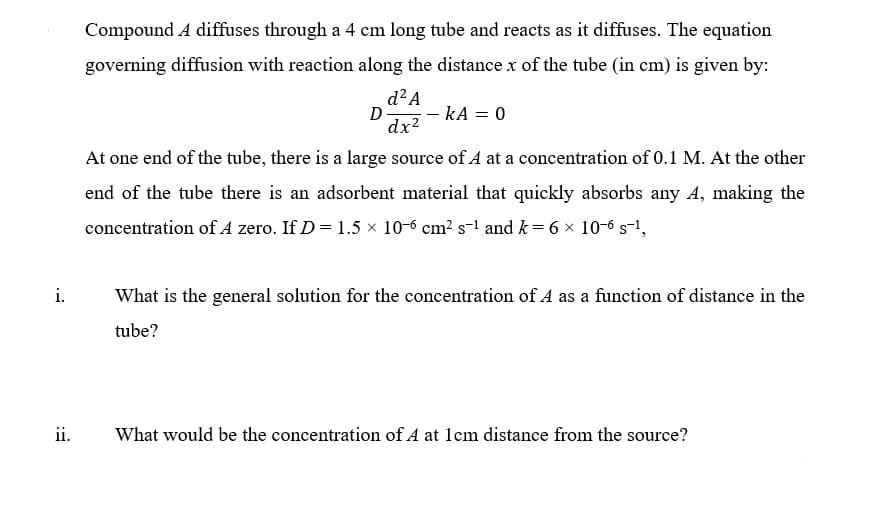
Transcribed Image Text:Compound A diffuses through a 4 cm long tube and reacts as it diffuses. The equation
governing diffusion with reaction along the distance x of the tube (in cm) is given by:
d²A
D
dx2
– kA = 0
At one end of the tube, there is a large source of A at a concentration of 0.1 M. At the other
end of the tube there is an adsorbent material that quickly absorbs any A, making the
concentration of A zero. If D = 1.5 x 10-6 cm? s-1 and k= 6 x 10-6 s-1,
i.
What is the general solution for the concentration of A as a function of distance in the
tube?
ii.
What would be the concentration of A at lcm distance from the source?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps
