Consider 0.900 mol of an ideal diatomic gas that undergoes the following processes: A-B: A compression from a volume of 12.00×10-3 m3 up to a volume of 8.00×10-3 m3 at constant pressure of 4.04×105 Pa. B-C: It expands following a process that is a straight line on the pressure-volume diagram up to a volume of 9.34 ×10-3 m3 reaching a pressure of 5.19×105 Pa. C-A: It expands isothermally until it reaches its starting point and closes the cycle. Use the diagram as reference Calculate the heat in each thermodynamic process and in the cycle.
Consider 0.900 mol of an ideal diatomic gas that undergoes the following processes: A-B: A compression from a volume of 12.00×10-3 m3 up to a volume of 8.00×10-3 m3 at constant pressure of 4.04×105 Pa. B-C: It expands following a process that is a straight line on the pressure-volume diagram up to a volume of 9.34 ×10-3 m3 reaching a pressure of 5.19×105 Pa. C-A: It expands isothermally until it reaches its starting point and closes the cycle. Use the diagram as reference Calculate the heat in each thermodynamic process and in the cycle.
Related questions
Question
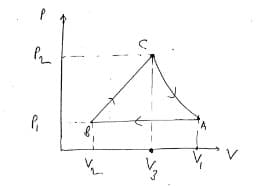
Consider 0.900 mol of an ideal diatomic gas that undergoes the following processes:
A-B: A compression from a volume of 12.00×10-3 m3 up to a volume of 8.00×10-3 m3 at constant pressure of 4.04×105 Pa.
B-C: It expands following a process that is a straight line on the pressure-volume diagram up to a volume of 9.34 ×10-3 m3 reaching a pressure of 5.19×105 Pa.
C-A: It expands isothermally until it reaches its starting point and closes the cycle.
Use the diagram as reference
Calculate the heat in each
Transcribed Image Text:لا
لیے
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps
