2.16 Consider a hypothetical X*-Y¯ ion pair for which the equilibrium interionic spacing and bonding energy values are 0.35 nm and –6.13 eV, respectively. If it is known that n in Equa- tion 2.11 has a value of 10, using the results of Problem 2.14, determine explicit expres- sions for attractive and repulsive energies EA and Er of Equations 2.8 and 2.9.
2.16 Consider a hypothetical X*-Y¯ ion pair for which the equilibrium interionic spacing and bonding energy values are 0.35 nm and –6.13 eV, respectively. If it is known that n in Equa- tion 2.11 has a value of 10, using the results of Problem 2.14, determine explicit expres- sions for attractive and repulsive energies EA and Er of Equations 2.8 and 2.9.
Related questions
Question
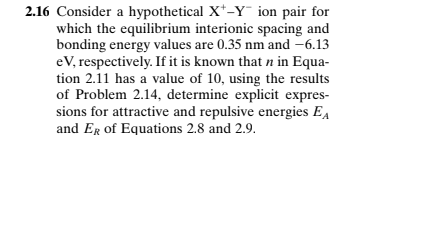
Transcribed Image Text:2.16 Consider a hypothetical X*-Y ion pair for
which the equilibrium interionic spacing and
bonding energy values are 0.35 nm and -6.13
eV, respectively. If it is known that n in Equa-
tion 2.11 has a value of 10, using the results
of Problem 2.14, determine explicit expres-
sions for attractive and repulsive energies EA
and Er of Equations 2.8 and 2.9.

Transcribed Image Text:A
rert))
renta)
nB
ren+z)
movo,
%3D
A
%3D
(Cm+a))
Cnte)
To
A
B
Eo
nB
Equation Summary
Equation
Number
Page
Number
Equation
Solving for
E = S F dr
Potential energy between
2.4
29
two atoms
Attractive energy between
2.8
31
two atoms
B
En =
Repulsive energy between
2.9
31
two atoms
{1 - exp[-(0.25)( xa – Xa)
2.10
%IC =
x 100
Percent ionic character
33
A
B
EN =
(2.11)
List of Symbols
Symbol
Meaning
A, B, n
Maieriai cosianis
E
E.
ER
F
Potential energy between two atoms/ions
Attractive energy between two atoms/ions
Repulsive energy between two atoms/ions
Force between two atoms/ions
Separation distance between two atoms/ions
Electronegativity value of the more electronegative
element for compound BA
Electronegativity value of the more electropositive
element for compound BA
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps
