Consider a sample of an ideal gas with 10 molecules in the sample. 580 J of heat is added to the sample quasi-statically. Answer questions below. Hint a. If the ideal gas is monatomic, how much does the temperature of the gas rise, if: o the gas is heated at constant volume? Temperature rises by 20.02 X K. o the gas is heated at constant pressure? Temperature rises by b. If the ideal gas is diatomic, how much does the temperature of the gas rise, if: o the gas is heated at constant volume? Temperature rises by K. K. o the gas is heated at constant pressure? Temperature rises by K. c. For monatomic gas, how much work is done by the gas as 580 J of heat is added while the gas remains at constant pressure? Hint for (C) Monatomic gas J of work as 580 J is added at constant pressure.
Consider a sample of an ideal gas with 10 molecules in the sample. 580 J of heat is added to the sample quasi-statically. Answer questions below. Hint a. If the ideal gas is monatomic, how much does the temperature of the gas rise, if: o the gas is heated at constant volume? Temperature rises by 20.02 X K. o the gas is heated at constant pressure? Temperature rises by b. If the ideal gas is diatomic, how much does the temperature of the gas rise, if: o the gas is heated at constant volume? Temperature rises by K. K. o the gas is heated at constant pressure? Temperature rises by K. c. For monatomic gas, how much work is done by the gas as 580 J of heat is added while the gas remains at constant pressure? Hint for (C) Monatomic gas J of work as 580 J is added at constant pressure.
Related questions
Question
7
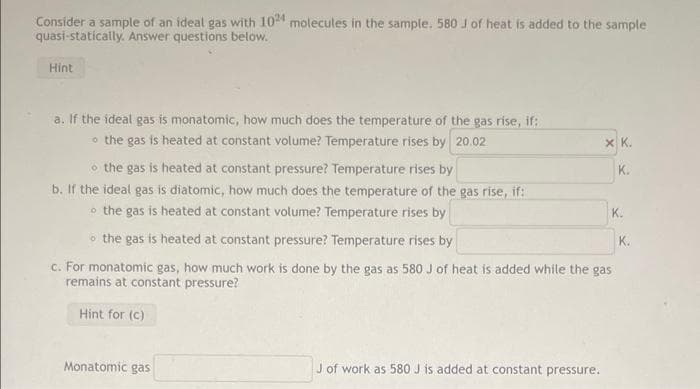
Transcribed Image Text:Consider a sample of an ideal gas with 10 molecules in the sample. 580 J of heat is added to the sample
quasi-statically. Answer questions below.
Hint
a. If the ideal gas is monatomic, how much does the temperature of the gas rise, if:
o the gas is heated at constant volume? Temperature rises by 20.02
X K.
o the gas is heated at constant pressure? Temperature rises by
b. If the ideal gas is diatomic, how much does the temperature of the gas rise, if:
o the gas is heated at constant volume? Temperature rises by
K.
K.
o the gas is heated at constant pressure? Temperature rises by
K.
c. For monatomic gas, how much work is done by the gas as 580 J of heat is added while the gas
remains at constant pressure?
Hint for (c)
Monatomic gas
J of work as 580 J is added at constant pressure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
